Full text
advertisement

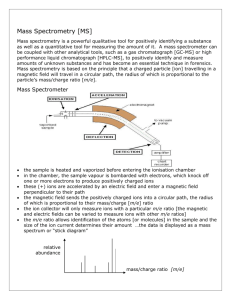
Development of CE-based Methods for the Analysis of Technical Surfactant Samples Bachelor Project, BSc Chemistry B.W.J. Pirok Summary of Contents Abstract ....................................................................................................................................... 3 1 Introduction ............................................................................................................................. 4 2 Capillary Electrophoresis ........................................................................................................... 5 2.1 Principles ...................................................................................................................................... 5 2.2 Electro-osmosis ............................................................................................................................ 6 2.3 Ionic Mobilities ............................................................................................................................. 7 3 Detection ................................................................................................................................. 9 3.1 UV Absorption Detection .............................................................................................................. 9 3.2 Indirect Detection........................................................................................................................ 10 3.3 Time of Flight Mass Spectroscopy Detection .............................................................................. 11 4 Experimental .......................................................................................................................... 13 4.1 Apparatus ................................................................................................................................... 13 4.2 Chemicals.................................................................................................................................... 13 4.3 Procedures .................................................................................................................................. 13 4.4 Methods ..................................................................................................................................... 14 5 Results ................................................................................................................................... 15 5.1 UV Absorption Detection............................................................................................................ 15 5.2 Indirect Detection ....................................................................................................................... 17 5.3 Time of Flight Mass Spectrometry Detection ............................................................................. 20 5.4 Conclusions ................................................................................................................................. 22 6 Discussion .............................................................................................................................. 23 Achknowledgements ......................................................................................................................... 23 References ................................................................................................................................. 24 ATTACHMENTS........................................................................................................................... 25 Sample 93 .......................................................................................................................................... 26 Sample A1 .......................................................................................................................................... 27 Sample D3.......................................................................................................................................... 29 Sample H1.......................................................................................................................................... 31 Sample J1 ........................................................................................................................................... 33 Sample W0 ........................................................................................................................................ 36 2 Abstract Technical anionic surfactant samples were separated by capillary electrophoresis using, time-of-flight mass spectroscopy and direct and indirect UV detection as analysis technique. Fifteen out of sixteen samples provided were divided into five classes based on experimental data. One sample was found to be unique. Six samples were found to be alcohol propoxylate based, ten samples were olefin based. Disulfates were also found for some of the alcohol propoxylate samples. Samples D3, D4 and DD were found to be unique. A molecular structure was proposed for components found in alcohol propoxylate samples. 3 1 Introduction The goal of the project was to develop methods, based on capillary electrophoresis, for the characterisation of technical surfactant samples. Sixteen different samples were provided by Shell Amsterdam. The samples were alcohol propoxylate sulfate and olefin sulfonate products with different alkyl chain lengths, with distributions varying from about C12 to C30. For most of the samples an active material percentage value was provided. Differences in viscosity, colour and active material concentrations were observed and used to divide the samples in groups showing similar external characteristics. The information provided indicated that the samples were expected to contain surfactants that contained no chromophoric groups. As such, the surfactants were not expected to absorb UV light. Together with the absence of context information regarding the samples, this complicated the development of a method for analysis. This thesis will summarize the project’s results. First, the theoretical background will be explained, by giving an overview of the principles of capillary electrophoresis, followed by an overview of the different detection methods and the possibilities for their application for the analysis of the samples. Second, the experimental section will specify the developed methods used to analyse and prepare the samples, including other relevant data. Next, the results will be presented for all investigated detection techniques, followed by a discussion in which the complications presented by the characteristics of the samples and their consequences for method development will be addressed. 4 2 Capillary Electrophoresis 2.1 Principles Electrophoresis is the migration of charged particles in a solution under influence of an electric field. The size and/or charge determine the velocities at which different particles migrate, which is the basic principle of all electrophoretic separation methods.1 The electrostatic force 𝐹 exerted on a particle 𝑖 in a solution is proportional to the net charge of the particle 𝑞𝑖 and the electric field strength 𝐸 in the solution: 𝐹 = 𝑞𝑖 ∙ 𝐸 The direction of the electrostatic force is to the electrode with an opposite charge than that of the particle itself. The influence of the electrostatic force causes a charged particle to accelerate and to start migrating, whilst being opposed by viscous forces in the solution, which increase proportional with the velocity 𝑣𝑖 of the particle. The Stokes equation gives the viscous force for a spherical particle Figure 1 – Basic CE setup 𝐹 = 6𝜋𝜂 ∙ 𝑟𝑖 ∙ 𝑣𝑖 where 𝜂 is the viscosity of the solution and 𝑟𝑖 the effective radius of the particle. The opposing forces cancel each other out after a very short acceleration time. The particle then moves with a constant velocity through the solution: 𝑣𝑖 = 𝑞𝑖 ∙ 𝐸 6𝜋𝜂 ∙ 𝑟𝑖 Different ions can be separated when having different charge, radius or a different charge/size ratio. In order to easily compare experimental data obtained with different field strengths, the ionic mobility 𝜇𝑖 has been defined as: 𝜇𝑖 = 𝑣𝑖 𝐸 with a dimension of m2 V-1 s-1. When combining the above two equations it follows that the mobility of a spherical particle can be written as: 𝜇𝑖 = 𝑞𝑖 6𝜋𝜂 ∙ 𝑟𝑖 As such it is clear that different ions can be separated when they differ in charge or radius or when their ratio differs. For mobility the radius of the moving particle in aqueous solution, including its hydration shell, determines the mobility of the particle. The Ion H+ Li+ K+ Mobility (m2 V-1 s-1) 362 40 76 mobility of a K+ ion is for example larger than the much smaller Table 1 – Mobilities of some inorganic ions Li+ (Table 1), as it binds more water molecules. The much higher in water at infinite dilution. mobility of a H+ ion, however, has little to do with the relative 5 small size of a bare proton as its hydrated shell is much larger compared to other simple inorganic ions in aqueous solution. In fact the proton has interactions in the form of hydrogen bonds with surrounding water molecules. Therefore simple rearrangements in the electronic structure throughout the solution may transport protons and since there is no real mass transport involved, the transport may take place at higher velocities relative to other simple inorganic ions. Ionic Field Strength The equations addressed thus far for mobilities of ions are only valid in very dilute solutions, where the ionic strength approaches zero. In CE, a finite salt concentration will be presented as background electrolyte (BGE) in the solution. In an electrolyte solution an ion will be surrounded by other ions, which together form a diffuse cloud of ions oppositely charged to the surrounded or central ion, so that they neutralize the charge of the central ion.2 When increasing the ionic strength of the solution, this cloud will be much denser. As the diffuse ion cloud has a charge opposite to that of the central ion, it will migrate in the opposite direction during the electrophoresis process. This creates an extra viscous force exerted on the central ion, which becomes stronger as the ionic field strength of the solution increases and is called the electrophoretic effect. In addition, when the central ion and the diffuse ionic cloud have moved in opposite directions, they will attract each other again due to electrostatic differences and the cloud will rearrange itself again around the central ion. This is called the relaxation effect. Both the electrophoretic and relaxation effect work in the same direction and the mobility of an ion decreases as the ionic strength of a solution is increased.3 This effect is larger for multiple-charged ions, changing the ionic strength of the BGE therefore may help to separate overlapping zones of differently charged analytes. 2.2 Electro-osmosis In electrophoresis an aqueous solution of electrolytes is used and in contact with the wall of the capillary. (Fig. 2) The fused silica wall of a capillary contains negatively charged SiO- groups. This causes a charge separation between the wall and the solution as the electrolyte solution is positive, due to the electro neutrality principle. This excess of positive ions is electrostatically attracted by the negative wall. Figure 2 – Schematic of the capillary wall, showing the principles of electro-osmosis. (Reproduced from W.Th. Kok, 1999) 6 Applying voltage between the ends of the capillary results in the electric field to exert a force on the excess positive charge present in the solution, close to the wall, driving the solution towards the negative electrode. The viscous forces in the thin layer of solution near the wall will counteract the electrostatic force, rendering a constant flow of the solution and is called electro-osmosis. This principle is an important feature of CE as the separation is performed in free solution. Since the velocity of this electro-osmotic flow (EOF) is proportional to the field strength, the electroosmotic mobility 𝜇𝑒𝑜 can be defined in a way that is similar to the definition of the ionic mobility. The electro-osmotic mobility depends on the charge density on the wall, which is determined mainly by the wall material and the pH of the solution. While present in a background electrolyte buffer that migrates towards the negative pole, anionic analytes will migrate toward the positive pole. Therefore, the electro-osmotic mobility is expected to be higher than that of the anionic analytes present in the technical samples. When introducing a sample at the positive end of the capillary, with the detector at the negative end, the solutes may be determined in a single run. From the net velocity of an ion, as obtained from the length of the capillary up to the detector position 𝐿𝑑 and the migration time 𝑡𝑖 , the apparent mobility 𝝁𝒂𝒑𝒑,𝒊 can be calculated: 𝜇𝑎𝑝𝑝,𝑖 = 𝐿𝑑 𝑡𝑖 ∙ 𝐸 It is important to note that as the electrostatic force acts only on the outer layer of the solution, with its excess of positive ions, nearly the entire solution moves with the same speed, except for a very thin layer of a few nm close to the wall. This implies that the electro-osmotic flow does not cause zone broadening. When comparing electropherograms of two different runs of the same sample, the mobilities of the electro-osmotic flow and the analytes may differ because of slight changes in the surface of the wall of the capillary. Through subtraction of the electro-osmotic mobility from the apparent mobility of particle 𝑖, the effective mobility of the particle may be obtained: 𝜇𝑒𝑓𝑓,𝑖 = 𝜇𝑎𝑝𝑝,𝑖 − 𝜇𝑒𝑜 2.3 Ionic Mobilities For the design of a CE method it is useful to estimate the expected ionic mobilities of the analytes on forehand. Any differences in observed values compared to those expected may then be addressed and will ensure that supposedly known or provided information will be regarded with a critical eye. When the equivalent ionic conductivity of particle 𝑖 is known, the ionic mobility 𝜇𝑖 may easily be calculated using the Faraday constant 𝐹 through: 𝜇𝑖 = 𝜆0,𝑖 𝐹 For electropherogram interpretation it is useful to be aware of the different parameters that may influence the mobility. The remainder of this paragraph will be address these parameters. 7 Of course the mobility of an ion is influenced by its charge. An ions mobility therefore is expected to be proportional to its charge number z, but generally the mobility of a multiple-charged ion is less than z times that of a singly-charged ion of similar size. This effect is also observed for compounds that may have a different charge depending on the pH of the solution and is stronger for multiplecharged ions that have the charges located on the same functional group. Furthermore a higher ionic strength of the solution will increase this observed effect as the retardation effect, mentioned in the previous paragraph, will be stronger for higher charged compounds. It is clear, from the equation for the mobility of a spherical particle from paragraph 2.1, that the mobility is inversely proportional to its molecular weight. So a larger molecular weight will render a lower mobility of the ion. For the provided technical samples, distributions in chain length to the present surfactants are expected. A longer chain length equals a higher molecular mass and thus a lower mobility of the ion. In practice molecules with the same charge and similar molecular weight may often easily be separated. Indeed, it follows that the decisive influence of the mobility of an ion is a result from its chemical structure. This influence is hard to predict, but several factors may be of importance. First of all, the type of charged group may be of importance. Despite their higher molecular weight, sulfonic acids and sulphates have higher ionic mobilities than the corresponding carboxylic acids. Cations often have a higher mobility than anions with the same molecular weight. Secondly, the substitution of extra alkyl groups in the structure decreases the mobility of an ion similarly to that expected of the increase in molecular weight. Third, the substitution of a hydroxyl group may be very small or even render an increase of the ionic mobility instead. Finally, hydrophobic groups will render a smaller decrease in ionic mobility when substituted, compared substitution of a methyl group. 8 3 Detection 3.1 UV Absorption Detection The measurement of the absorption of light in the UV region is the standard method of detection for capillary electrophoresis. The detection may be performed directly on-column and no physical contact between the detection system and capillary is needed which reduces zone broadening. This absence of contact with the separation medium also allows for high voltages to be used during separation, as the medium is not in contact with the sensitive equipment. The capillaries used are made from fused silica, a quartz-like material which is transparent for light with wavelengths down to 190nm. This makes the capillary itself a perfect optical cell. For most samples a colour in the range of yellow to brown was observed, which indicated that they should show absorption in the UV spectrum.1 The detector produces a signal, the absorbance 𝐴, that is obtained through the molar absorptivity of the analyte 𝜀, its concentration 𝑐 and the effective length of the light path through the solution 𝐿𝑒𝑓𝑓 : 𝐴 = 𝜀 ∙ 𝑐 ∙ 𝐿𝑒𝑓𝑓 Following from this definition, the sensitivity (𝐴/𝑐) for a particular compound is proportional to 𝐿𝑒𝑓𝑓 . Since the light is measured in a direction perpendicular to the capillary axis, 𝐿𝑒𝑓𝑓 can be no more than the capillary diameter. This explains why UV absorption measurements with CE have limits of detection that are approximately two orders of magnitude higher than in HPLC. Yet this may be compensated by using a less low wavelength, where many compounds show high absorptions. Finally, it is important to note that the UV detection is performed on an optical cell that has a cylindrical shape.4 Illumination of the cell by a parallel beam of light with a certain width (𝑤𝑏 ) results in a varying length of light over the width of the beam. Aside from the capillary inside diameter, the effective light path length 𝐿𝑒𝑓𝑓 depends also on the ratio between the light beam width and the capillary inside diameter. When 𝑤𝑏 is exactly as wide as the capillary inside diameter, the path length varies from 0 at the top and the bottom, to 𝑑𝑐 in the middle of the capillary cross-section. When the light beam is wider than the capillary inner diameter, part of the light will simply pass the capillary undisturbed by the absorbance in the solution. This will decrease the sensitivity of detection since the signal produced by the UV detector is based on a comparison of the intensity 𝐼 of the light beam passing the optical cell with a reference value 𝐼0 .5 The ratio of 𝐼 and 𝐼0 is translated electronically into the signal 𝐴: 𝐴 = − log 𝐼 𝐼0 The diode-array detector (DAD) allows a part of the spectrum where no absorbance is expected to be used for reference. Using this, when due to insufficient focusing of the beam a part of the light then passes the capillary undisturbed, the amount of light absorbed by compounds in the solution is not affected. Yet, relative to the total intensity, the change of 𝐼 by absorption will be smaller and the lower change of the ratio between 𝐼 and 𝐼0 will then be translated into a lower signal.6 Even though the target surfactants in the technical samples are not expected to contain any functional groups, a colour could be physically observed and hinted at the presence of absorbing 9 material. This could be unreacted material used to create the surfactants, but also could have been an unexpected feature of the surfactant molecules. Henceforth, UV absorption measurements were taken of all sixteen samples. 3.2 Indirect Detection An attractive feature of CE was the possibility to apply indirect detection on analysis of the surfactants, as they were expected to show have absorption themselves.7 For indirect detection, an ionic compound with a high UV absorbance is used as one of the constituents of the background electrolyte solution and functions as monitoring ion.8 Using a monitoring ion results in an electropherogram with a background absorption. When an analyte in such a solution passes through the detector, the local concentration of the monitoring ion will be reduced, resulting in a decrease of absorption. As such indirect detection is universal and non-selective. In order to make a founded choice of the operating conditions, the transfer ratio for a specific analyte-BGE combination should be considered.9 This ratio may be defined as: 𝑇𝑅 = − ∆𝑐𝑎 𝑐𝑖 Here, ∆𝑐𝑎 is the change in concentration of the monitoring species by the analyte concentration 𝑐𝑖 in a zone. The minus sign indicates that the changes in absorption are expressed as decrease in the baseline signal. Since the changes of 𝑐𝑎 are being monitored, the transfer ratio is a measure of the sensitivity that will be obtained. In indirect detection, the quantitative reliability for low analyte concentrations is often not so much limited by detector noise but rather by instabilities of the baseline. Instabilities as baseline drifts and shifts and the appearance of unexpected often broad peaks in the electropherogram may interfere with the recognition and integration of the analyte peaks. These baseline instabilities are often repeatable and seem to be unrelated to the injected sample. Even injecting no sample at all may results in the same baseline pattern after switching on the high voltage source. These baseline instabilities can be explained through temperature differences over the length of the capillary.10 The disturbance of the monitoring ion concentration visible on the electropherogram can come from both ends of the capillary to the detection window. It is impossible to eliminate all the thermal nodes through with these disturbances could occur. Yet the number of disturbances coming is related to the number of components in the BGE composition. These disturbances will from now on be referred as system peaks. To keep the number of system peaks low and improve the selectivity of the separation, the number of ionic components should be kept to a minimum. To analyse the technical samples using indirect UV detection, sorbate and benzoate were investigated as monitoring ions and each used during measurement of all sixteen samples. In order to investigate the identity of observed phenomena on electropherograms obtained, the samples were spiked with standard compounds. These standard compounds were a sulfonate and sulfate like the surfactants, but have a fixed and, more importantly, known alkyl chain length. The electropherograms of the standards could then be cross-referenced with those of the samples, providing information about the identity of observed phenomena. Lauryl sulfate (C12) and hexane sulfonate (C6) were used to spike the samples. 10 3.3 Time of Flight Mass Spectroscopy Detection To be able to obtain more information on the technical samples, a time of flight mass spectrometer (TOF-MS) was coupled with the CE instrument using an interface with the electrospray (ESI) ionization ion source where the sample is introduced into the TOF analyser. Electrospray Ionization Electrospray is produced by applying a strong electric field, under atmospheric pressure, to a liquid passing through a capillary tube with a weak flux. By applying a potential difference of 4 kV between this capillary and the counter electrode, the electric field is obtained. This field induces a charge accumulation at the liquid surface located at the end of the capillary, and explodes to form highly charged droplets. A gas injected coaxially at low flow rate allows the dispersion of the spray to be limited in space. These droplets then pass either through a curtain of heated inert gas, most often nitrogen. At onset voltage the pressure is higher than the surface tension, the shape of the drop changes at once to a Taylor cone and small droplets are released, which divide and explode, producing the spray. This spray in turn consists out of very small highly charged droplets that continue to lose solvent and accumulate charge. When the electric field on their surface becomes large enough, desorption of ions from the surface occurs. Because of limitations from the electrochemical process, the electrospray current is limited. This process occurs at the probe tip and is sensitive to concentration rather than to total amount of sample. Time of Flight Mass Spectroscopy To couple the continuous ion beam from the ESI with the TOF analyser, orthogonal acceleration was used (Fig. 3). As the sample was continuously ionized in the ion source, the parallel beam passes an RF octapole ion guide that transports the ions efficiently to the orthogonal accelerator. This not only focuses the ions from the source but also controls the kinetic energy of these ions by collisional cooling. The resulting beam is directed to the orthogonal accelerator and continuously fills the first stage of the ion accelerator in the space between a plate (depicted in figure 3 as a red line) and a grid (purple) at 0 V. Initially, the voltage on the plate is 0 V like the grid. So the region is field free and ions continue to Figure 3 – Simplified schematic of a TOF-MS instrument. move in their original direction. Then an injection pulse voltage of 𝑉𝑖𝑛𝑗𝑒𝑐𝑡𝑖𝑜𝑛 is applied to the plate. The ions between the plate and the grid are pushed by the resulting electric field in a direction orthogonal to their original trajectories and start flying to the analyser. After passing through the grid the ions are accelerated more towards a second grid (blue) which is at a voltage of 𝑉𝑇𝑂𝐹 . Then the ions enter the field-free drift region at a potential of 𝑉𝑇𝑂𝐹 where the TOF mass separation occurs. 11 Once all ions moved to the field-free region through the second grid, the voltage on the plate is restored to 0 V and ions from the ion source will begin to fill the space between the plate and the first grid again. Thus during the time that the ions continue their flight in the field-free region, the orthogonal accelerator is refilled with new ions. One flight cycle will end when the ion with the highest m/z reaches the detector. Another flight cycle will begin by reapplying a pulse voltage to the plate. As such the distance travelled by the ion beam between two successive injection pulses in the first stage of the orthogonal accelerator is the same as the distance travelled by the ion in the flight tube between the orthogonal accelerator aperture and the detector. It should be noted that the time required for the ion beam to fill the orthogonal accelerator is lower than the time for sampled ions to fly to the detector. Therefore a part of the ions produced in the source are not pushed to the flight tube and are lost in the first stage of the orthogonal accelerator. Reflectron The reflectron is located behind the field-free region opposed to the ion source. The detector is located on the source side of the ion mirror and captures the arrival of ions reflection by the reflectron. This reflectron corrects the kinetic energy dispersion of the ions leaving the source with the same m/z ratio. Ions with more kinetic energy and hence more velocity will penetrate the reflectron more deeply than ions with lower kinetic energy. Consequently, faster ions will spend more time in the reflectron and will reach the detector at the same time than slower ions with the same m/z. Although the reflectron increases the flight path without increasing the dimensions of the mass spectrometer, this positive effect on the resolution is of lower interest than its capability to correct the initial kinetic energy dispersion. However, the reflectron increases the mass resolution at the expense of sensitivity and introduces a mass range limit. Coupling of a CE instrument In CE, the flow rate of the buffer in the capillary, caused by the generation of the electro-osmotic flow, is very low, on the order of several hundred nl/min. For this reason, in order to conduct stable CE/MS analysis, the system is devised so that a sheath liquid is sent from outside the capillary to supplement the flow rate (Fig. 4). Furthermore, the sheath liquid serves to electrically connect the CE outlet to the ground potential at the sprayer, similar to the function of the outlet buffer in a CEonly system. During these experiments a LC pump with a splitter was used to obtain a sheath flow of 0.4ul/min. Figure 4 – Schematic of the spray needle used to couple the CE instrument with the TOF-MS. 12 4 Experimental 4.1 Apparatus Two Agilent G1600AX HP 3D CE systems were used throughout the experiment, both with a standard setup using an UV detector for analysis. One for direct UV detection and one for indirect UV detection. At a later stage the CE instrument used for direct UV detection was coupled to a Agilent G1969A LC/MSD TOF instrument. The TOF instrument had an electrospray ionization ion source installed. An Agilent 1100 Series Isocratic Pump and Degasser were used to provide the sheath liquid at a constant flow (Fig. 5). The isocratic pump had an 1:100 splitter installed to be able to produce sheath flows on the order of µl/min. 4.2 Chemicals Background Electrolyte For direct detection, a 5 mM ammonium acetate solution was used as BGE. For indirect detection a solution of 5 mM borax and 10 mM sorbate was used as BGE, where sorbate functioned as monitoring ion. Both solutions were made using methanol/water (8:2) as solvent. For CE-TOF-MS analysis the BGE consisted of 5 mM ammonium acetate also using methanol/water (8:2) as solvent. Capillary Fused silica capillary with an internal diameter of 75 µm was used during all analysis. For direct and indirect UV detection the capillary had a total length of 50 cm, with a length to the detector of 41.5 cm. For CE-TOF-MS analysis the Original Code % AM Type Viscosity Colour Class capillary had a total length of 80 973 93 35,4 OS Low Yellow W cm, with a length of 21.5 cm to A771 A1 30,5 APS High Colorless D3 72,4 OS Very Low Light Brown D the UV detector of the CE Drum 3 Drum 4 D4 72,4 OS Very Low Light Brown D instrument and the full 80 cm to Drum D DD 28,0 OS Very Low Creme D the TOF-MS instrument. Drum Q DQ 33,0 OS Low Yellow W Used Chemicals All chemicals used were obtained from standard supplies. Samples Sixteen samples were provided for analysis. Details have been layed out in table 2. H771 H771-2 J13131 J13131-2 J771 WFE10 #1 #5 #7 #11 H1 H2 J1 J2 J7 W0 X1 X5 X7 X11 30,8 26,0 34,7 28,1 28,9 35,5 - APS APS APS APS APS OS OS OS OS OS High High High High High Low Very Low Very Low Very Low Very Low Colorless Colorless Colorless Colorless Colorless Brown Light Yellow Yellow Brown Dark Brown H H J J J W X X X X Table 2 – Detailed information on provided technical samples. (APS = Alcohol propoxylate sulfate, OS = olefin sulfonate) 4.3 Procedures Preconditioning Since onward analysis revealed that the capillaries could clot due to some of the samples a special preconditioning program was executed at the start of each day. The program consisted of flushing the capillary first 15 minutes with acetonitrile, followed by 15 minutes of 0.1M sodium hydroxide solution and finally 15 minutes with water. 13 Sample Preparation Since for 12 samples the active material (AM) concentration was provided (Table 2), an amount of these sample was added to 10 mL water/methanol (8:2) until an AM concentration of 30% was reached. For the remaining four samples the AM concentration was estimated based on their observed densities, after which they were dissolved to a obtain an AM concentration similar to 30%. 4.4 Methods Direct and indirect UV Absorption Detection Measurements Each measurement run started with a preconditioning program where the capillary was flushed with acetonitrile for 2 min, 0.1M sodium hydroxide solution for 2 minutes and BGE solution for 2 minutes. Next, the sample was injected by a pressure of 30 mbar/4 sec after which the run started by applying a voltage of 30 kV during the acquisition time of 30 min. The CE was set to run electrically in the positive mode. The cassette was kept at a temperature of 25 oC during the entire time. Wavelengths of 200nm and 254 were observed during each run with a response time of 0.2sec. CE-TOF-MS Measurements On the CE side of the system: Each measurement run started with a preconditioning program where the capillary was flushed with BGE for 4 min. Next, the sample was injected by a pressure of 30 mbar/3 sec after which the run started by applying a voltage of 30 kV during the acquisition time of 30 min. The CE was set to run electrically in the positive mode. The cassette was kept at a temperature of 25 oC during the entire time. Wavelengths of 200nm and 254 were observed during each run with a response time of 0.2sec. The isocratic pump was set to provide a flowrate of 0.4 ml/min, with the 1:100 splitter this resulted in a flow of 4µl/min sheath flow. The TOF-MS instrument had its ion polarity set to negative. The gas temperature was set to 300 oC. The drying gas ran at a flow of 7 l/min with the nebulizer at 15 psig. A voltage of 4 kV was applied to the capillary and the fragmentor had a voltage of 200 V. Figure 5 – Schematic of the setup used to couple the CE instrument with a TOF-MS instrument. 14 5 Results Electropherograms were successfully obtained in both direct and indirect mode for each of the samples. In order to identify possible system phenomena a blank was taken for each mode as well. System and standard phenomena were compared with the sample electropherograms using ionic mobility calculations for each of them. During sample preparation a notable difference in solubility and viscosity of the samples was observed. Samples H1, H2, A1, J1, J2 and J7 were much easier to dissolve in water than the remaining ten samples. Furthermore, no colour was observed for the six samples mentioned, whereas the remaining 10 were all showing colours in the range of crème-yellow to yellow-brown. These observations indicated that the properties of the six samples mentioned featured notable difference compared to those of the remaining ten. 5.1 UV Absorption Detection Experimental data taken in the direct mode revealed a close relation between certain samples, which allowed classification. For samples D3, D4 and DD a near perfect correlation was observed, both in peaks and intensity of the peaks (Fig. 6a). Mobility calculations (paragraph 2.2) performed on the largest peak visible on each of the three electropherograms confirmed that they were all the same and the samples were grouped as Class D. Indeed, a large shift can be observed for the green line which represents sample DD. This shift, however, is also visible for the electro-osmotic flow peak. As explained in paragraph 2.1, to compare a peak observed on one electropherogram with one observed on another, one must compare the mobilities for both peaks. These mobilities are first corrected by the mobility of the electro-osmotic flow. Figure 6 – Overlays of electropherograms taken using direct UV detection at 200nm. a) Samples D3 (blue), D4 (red) and DD (green) at 200 nm. b) Samples 93 (blue), DQ (red) and W0 (green). S stands for system peak, EOF for acetone, representing the peak of the electro-osmotic flow. 15 The electropherograms taken for samples 93, DQ and W0 showed many similarities (Fig. 6b), which had no correlation with electropherograms taken from other samples. The peak marked with x proved to be not reproducible and was in fact an artefact. It can be seen that the intensity of the peaks observed for sample W0 (green) are slightly higher than those for the other two samples. Slight differences in peak shapes can be observed, but mobility calculations confirm that the relative locations are nearly identical. These samples were grouped and will from now on be referred to as class W. Direct UV detection performed on samples J1, J2 and J7 revealed a gathering of individual components to be observed for all samples (Fig. 7a). Highest intensities were observed for sample J1, whereas intensities were somewhat lower for sample J2 and J7. The spike observed for sample J2 at t = 5.6 was not reproducible on later runs and included here as the runs were all taken on the same sequence. A notable difference of the electropherograms of these three samples compared to those of the previous six was that the intensities of the observed peaks were significantly lower. This supports the preliminary observation that the properties of these samples were notable different than those of the previous two classes. The three samples were grouped as class J. Samples H1, H2 and A1 proved to be difficult to analyse using CE. Large baseline instabilities were observed on repeated measurements of all three samples (Fig. 7b), with odd phenomena to be observed for some of the runs. The significant absorption for sample A1 around t = 6.7, was consistently observed whereas the electropherograms of samples H1 and H2 did not show this increase. The broad peak observed on the electropherogram of sample H2 is the same as the small broad peak for sample H1, yet not as intense as it is for H2. Samples H1 and H2 correlated quite well and were grouped as class H. Figuur 7 – Overlays of electropherograms taken using direct UV detection at 200nm. a) Samples J1 (blue), J2 (red) and J7 (green) at 200 nm. b) Samples H1 (red), A1 (blue) and H2 (green). 16 Direct UV detection analysis of samples X1, X5, X7 and X11 revealed little useful information. The electropherograms correlated very well. Yet due to the absence of information regarding active material concentrations it was not possible to compare peak intensities. 5.2 Indirect Detection Both sorbate and benzoate were investigated as monitoring ions on standard compounds. Comparison of the obtained electropherograms showed that the performance of benzoate was poor compared to that of sorbate, as such sorbate was used to perform indirect detection on all sixteen samples. To be able to get an indication on the molecular size of sample compounds, several compounds of similar structure as the expected sample compounds, were investigated to be used as standard reference. Indirect UV detection measurements were performed for hexane sulfate, lauryl sulfate and tetradecyl sulfonate (Fig. 8). These compounds have an alkyl chain length of respectively six (C6), twelve (C12) and fourteen (C14) carbon atoms. Tetradecyl sulfonate turned out to perform poorly as standard compound and was discarded. Hexane sulfate and lauryl sulfate were used to spike the sampled to be measured with indirect UV detection. Comparison with the electropherograms obtained from analysis of a blank run of the background electrolyte buffer allowed identification of the system peaks. Figure 8 – Electropherogram obtained by indirect UV detection performed on hexane sulfate, lauryl sulfate in 5 mM sodium tetraborate decahydrate and 10 mM sorbate as BGE buffer at 220nm. For each class one sample was selected to represent the other samples of the class. Each of these samples was spiked with the two standard compounds, after which indirect UV analysis was performed. For every obtained electropherogram, two peaks were identified as the standard compound through comparison of effective mobility data from the electropherograms of the standards and analysed sample. An electropherogram of sample D4 spiked with the two standard compounds was obtained using indirect UV detection. Two peaks could successfully be identified as standard compound. Irregular peak shapes complicated phenomena classification as baseline instability, system peak or analyte peak. A wide peak was observed from t = 16.5 until t = 20.5 and identified as the peak at t = 6.5 on the direct UV detection electropherogram of sample D4 (Fig. 9). The shape of this peak was interpreted as being caused by the absorption of compounds reaching a concentration at the UV detector that was higher than that of the monitoring ion. 17 Figure 9 – Indirect UV detection electropherogram of Sample D4 in 5 mM sodium tetraborate decahydrate and 10 mM sorbate as BGE buffer at 220nm. Phenomena denoted S are system peaks. Indirect UV detection analysis of sample X7 provided little information as no clear specific phenomena could be observed, though standard compounds were correlated to two observed phenomena. (Fig. 10a) The broad peak observed at t = 15.5 was identified to be the same as peak observed on the direct UV detection electropherogram, similarly to sample D4. For sample W0 a broad valley was observed at t = 9.7 on the indirect UV detection electropherogram (Fig. 10b). This valley was also observed for samples 93 and DQ, supporting the earlier observation that samples 93, DQ and W0 belonged to the same class of samples. Figure 10 – Indirect UV detection electropherogram of a) sample X7 and b) sample W0 in 5 mM sodium tetraborate decahydrate and 10 mM sorbate as BGE buffer at 220nm. Phenomena denoted S are system peaks. 18 Similar to the direct UV detection analysis of sample H2, the indirect UV detection electropherogram showed a gathering of individual components. (Fig. 11) Standard compound peaks were successfully identified. The location of the remaining peaks relative to those of the standard compounds suggested that most peaks represented surfactant ions with an alkyl chain length being larger than 12 carbon atoms. The electropherogram showed that surfactants with chain lengths smaller than 12 carbon atoms appeared to separate better than those with longer chain length. The valley observed around t = 10 consisted out of multiple phenomena that merged into each other. One could also say that they did not separate well. At the same time information provided indicated that the minimum amount of carbon atoms expected in the variable alkyl chain would be 12. Whereas peaks were observed that would suggest an amount smaller than 6. The electropherogram interpretation was not consistent with information provided. A gathering of individual components was also observed for the electropherogram of indirect UV detection analysis of sample J2. Compared to the electropherogram of sample H2, the number of component phenomena observed was low. Also the intensities of the phenomena observed for sample J2 were lower than for sample H2. For these two sample classes, the electropherograms taken by indirect UV detection revealed that the area of C6 valley, which represented an amount smaller than 0.2mg ml-1 hexane sulfonate, was much larger than the area of the individual component valleys added up, while the sample concentration after dilution of a technical sample with a factor required to obtain an AM of 30%, was 12 mg ml-1. Hence, the observed surfactants did not account for the majority of the sample and thus were not strongly present in the pure sample. Figure 11 – Indirect UV detection electropherogram of a) sample H2 and b) sample J2 in 5 mM sodium tetraborate decahydrate and 10 mM sorbate as BGE buffer at 220nm. Phenomena denoted S are system peaks. 19 5.3 Time of Flight Mass Spectrometry Detection A TOF-MS instrument was successfully coupled to the CE instrument. In this setup, the length to the UV detector was merely 21.5 cm. So when the sample zone reached the UV detector, the sample had not enough time yet to separate. As such electropherograms obtained through the UV detector were only used as diagnostic tool. Total ion current (TIC) chromatograms were obtained through TOF-MS analysis of the samples. Each of them showed a peak at t=4, whilst the UV detector showed that the EOF should have been appearing around t=5.6. This meant that the observed peak at t=4 represented compounds that were faster than the EOF and thus must have been positively charged. The gap between each observed peak on the mass spectrum of these compound (Fig. 14b) was 82 m/z. This value corresponded with sodium acetate. Based on this information the observed phenomenon was classified as a system peak representing positively charged ion clusters, consisting of sodium acetate, as a result of the use of ammonium acetate as BGE. Class D and W Through analysis of the samples of class D, a TIC chromatogram was obtained that correlated well to the electropherogram obtained by direct UV detection analysis (Fig. 12). Mobility calculations allowed the conclusion that peak 4 in fact was the same as the large peak observed on earlier UV electropherograms. The peak was found to be consisting mainly of a compound with mass 377,27. TOF-MS analysis of class D samples showed that abundances and compounds found for all the samples were nearly identical. This information allowed the conclusion that all of the class D samples were nearly identical. Figure 12 – TIC chromatogram of sample D3. Samples 93, DQ and W0 were also found to be very similar based on information obtained through TOF-MS analysis. Some of the compounds found, having masses of 322,25 and 349,25 were also found during analysis of class D samples. The samples were found to be very similar in components observed on mass spectra, but their abundances were different. Class X As with UV detection, lack of information regarding active material concentrations hindered accurate abundance comparison of samples within the X class. Multiple gathering of individual components were observed for all the class X samples with similar masses (Fig. 13). Figure 13 – Mass spectrum of an observed phenomenon on the TIC chromatogram of sample X5. 20 Class H and J A TIC chromatogram and mass spectra were obtained through mass spectrometry detection applied on sample H1 (Fig. 14a). A high abundance phenomenon was observed from t = 8-8.5. The mass spectrum of this domain revealed a distribution being present (Fig. 14c). The larger gaps between the peaks represented a difference Figure 15 – Proposed molecular structure of a part of the of 58m/z, whereas the smaller gaps observed had surfactant molecule observed in distribution of sample H1. Presumably, the alkyl chain has a length of 12 or 13 carbon a difference of 14m/z. The information provided atoms (n = 12,13) and 0 to 10 propoxy groups (m = 0 – 10). indicated that sample H1 was a propoxylate product, a propoxy group has the mass of 58, explaining the observed distribution. The smaller gaps observed represented the addition or loss of a methyl group. The information pointed at a molecule (Fig. 15), consisting out of an alkyl chain of 12 or 13 carbon atoms and 0 to 10 propoxy groups. However, the intensities observed for an alkyl chain of 13 carbon atoms was found to be an odd number. Furthermore, this distribution was observed only for the broad peak on the TIC chromatogram. It was not observed on the mass spectra for the other peaks. Figure 14 – a) TIC chromatogram of sample H1. b) Mass spectrum of peak 1. c) Mass spectrum of peak 2. Area in green was used to substract background spectrum from peak spectra. 21 Analysis of the other peak shaped phenomena on the TIC revealed that peaks 3, 4 and 5 represented a compounds with a rounded m/z value of 291, 262 and 233 respectively. The difference between every following peak was constantly 29 m/z, exactly half of 58. This pattern was continued for the remaining peaks and hinted at the presence of a second distribution being present of the same compounds but with a second sulphate group attached to the molecule. When an ion is doubly charged the m/z value halves, thus gaps of half of the original mass value were expected. This would also explain why the doubly charged ions separate better than the singly charged ions from peak 2. Furthermore, it explained why there were valleys appearing on the indirect UV detection electropherogram near the hexane sulfate valley. As these valleys represented in fact disulfates, which are, being doubly charged, not of similar structure as hexane sulfate. Similarly, the separated compounds were also found for sample J1. The number of separated components and phenomena observed for sample J1 was, however, higher than for sample H1. Also the distribution found for sample J1 was present only at a higher mass range, indicating a different alkyl chain length. Sample J7 was notably different from J1 and J2: additional compound peaks were observed in the mass spectra that were also found for sample H1 and H2. Finally, sample A1 also showed the distribution with gaps of 58 and 14 m/z. A difference of 58 indicated a propoxy component, whereas 14 indicated a methyl component. This confirmed that samples A1, H1, H2, J1, J2 and J7 were in fact the alcohol propoxylate products. 5.4 Conclusions Experimental data allowed classification for fifteen of the sixteen samples into five distinct classes, two alcohol based, three olefin based. Samples H1 and H2 were very similar. Sample A1 was found to be unique, however, the combined distribution observed for samples H1 and H2, with gaps of 14 (methyl) and 58 (propoxy) was found for sample A1 too. This distribution was not found for class J samples. Class J samples had a distribution in the mass range of 700-900, with only gaps of 58 (propoxy), not 14 (methyl). Samples J1 and J2 were found to be very similar, sample J7 was notably different and contained compounds that were also found for samples H1 and H2. Samples A1, H1, H2, J1, J2 and J7 were all found to be alcohol propoxylate products. Class D, W and X samples were found to be olefin products. Sample D3, D4 and DD were found to be identical. Minor differences in compound abundances were found between samples 93, DQ and W0. No distributions were observed. The performance of CE in general was decent. Compounds and samples were successfully separated using developed methods, with disulfates naturally separating better than monosulfates. The presence of absorbing material hindered the use indirect UV detection. 22 6 Discussion With three different techniques of detection applied on the sixteen technical samples, three different types of information were obtained. This information was found to be difficult to relate as specified target information to be used for comparison were not always very clear. The low amount of background information complicated the analysis and interpretation of obtained data. Where indirect UV detection would have been ideal for analysis of these samples based on information provided, too much absorbing material of unknown identity was found to be significantly complicating analysis. Different monitoring ions were not found to be successful in overcoming this problem. Analysis by mass spectrometry was found to be more successful. Some components were identified, as for example in sample H1 (paragraph 5.3). Most components, however, have not entirely been identified and more investigation is required for identification. Data obtained for class X samples indicated the presence of a great number of components that were not observed for the other twelve samples, and require more investigation. Achknowledgements Thanks go out to prof. dr. ir. P. Schoenmakers for his insights in interpretation of some of the data, and prof. dr. S. van der Wal, for his help with the TOF-MS instrument. However, special thanks go to dr. W. Th. Kok, who guided me in this project. His insights and academic skills proved to be very helpful during critical moments of this project. A good mentor and teacher was found in him. 23 References (1) Kok, W. Capillary electrophoresis: Instrumentation and operation. Chromatographia 2000, 51, S5S89. (2) Harned, H. S.; Owen, B. B. In The Physical Chemistry of Electrolytic Solutions; Reinhold Publ.Corp.: New York, 1958; . (3) FRIEDL, W.; REIJENGA, J.; KENNDLER, E. Ionic-Strength and Charge Number Correction for Mobilities of Multivalent Organic-Anions in Capillary Electrophoresis. J. Chromatogr. A 1995, 709, 163-170. (4) Hjerten, S. Free zone electrophoresis. Chromatogr. Rev. 1967, 9, 122-219. (5) Xu, X.; Kok, W.; Poppe, H. Change of pH in electrophoretic zones as a cause of peak deformation. J. Chromatogr. A 1996, 742, 211-227. (6) Bruin, G. J. M.; Stegeman, G.; Vanasten, A. C.; Xu, X.; Kraak, J. C.; Poppe, H. Optimization and Evaluation of the Performance of Arrangements for Uv Detection in High-Resolution Separations using Fused-Silica Capillaries. J. Chromatogr. 1991, 559, 163-181. (7) Yeung, E. S.; Kuhr, W. G. Indirect Detection Methods for Capillary Separations. Anal. Chem. 1991, 63, A275-&. (8) Foret, F.; Fanali, S.; Ossicini, L.; Bocek, P. Indirect Photometric Detection in Capillary Zone Electrophoresis. J. Chromatogr. 1989, 470, 299-308. (9) Xiong, X.; Li, S. F. Y. Design of background electrolytes for indirect photometric detections based on a model of sample zone absorption in capillary electrophoresis. Journal of Chromatography a 1999, 835, 169-185. (10) Xu, X.; Kok, W. T.; Poppe, H. Noise and baseline disturbances in indirect UV detection in capillary electrophoresis. Journal of Chromatography a 1997, 786, 333-345. 24 ATTACHMENTS 25 Sample 93 Total Ion Current Chromatogram Mass Spectrum (Area 1) // EOF Mass Spectrum (Area 2) Mass Spectrum (Area 3) 26 Sample A1 Total Ion Current Chromatogram Mass Spectrum (Area 1) // EOF Mass Spectrum (Area 2) Mass Spectrum (Area 3) 27 Mass Spectrum (Area 4) Mass Spectrum (Area 5) Mass Spectrum (Area 6) Mass Spectrum (Area 7) Mass Spectrum (Area 8) Mass Spectrum (Area 9) Mass Spectrum (Area 10) 28 Sample D3 Total Ion Current Chromatogram Mass Spectrum (Area 1) // EOF Mass Spectrum (Area 2) 29 Mass Spectrum (Area 3) Mass Spectrum (Area 4) 30 Sample H1 Total Ion Current Chromatogram Mass Spectrum (Area 1) // EOF Mass Spectrum (Area 2) Mass Spectrum (Area 3) Mass Spectrum (Area 4) 31 Mass Spectrum (Area 5) Mass Spectrum (Area 6) Mass Spectrum (Area 7) Mass Spectrum (Area 8) 32 Sample J1 Total Ion Current Chromatogram Mass Spectrum (Area 1) // EOF Mass Spectrum (Area 2) Mass Spectrum (Area 3) 33 Mass Spectrum (Area 4) Mass Spectrum (Area 5) Mass Spectrum (Area 6) Mass Spectrum (Area 7) Mass Spectrum (Area 8) Mass Spectrum (Area 9) 34 Mass Spectrum (Area 10) Mass Spectrum (Area 11) Mass Spectrum (Area 12) Mass Spectrum (Area 13) 35 Sample W0 Total Ion Current Chromatogram Mass Spectrum (Area 1) // EOF Mass Spectrum (Area 2) Mass Spectrum (Area 3) 36