Pseudomonas Aeruginosa
advertisement

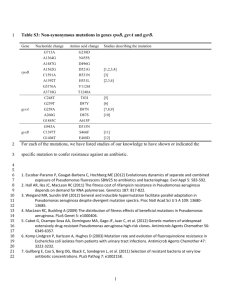
Biological sciences/11. Bioengineering and Bioinformatics c.b.s. Bekturova A.Zh., d.b.s. Khanturin M.R. L.N. Gumilev Eurasian National University, Kazakhstan Features of the bacteria Pseudomonas genomes organization and their hydrocarbon activity In the 50-60-th years of this century in the world and national literature appeared a large amount of research on bacteria Pseudomonas. Bacteria Pseudomonas are similar in morphology and very diverse in their cultural and physiological characteristics. The bacterial genome is represented by a single closed circular DNA molecule (bacterial chromosome), its size and composition in different strains are special. For example, the genome size of P. fluorescens Pf-5 is 7.1 m. bases pairs, and the strainPseudomonas fluorescens PfO-1 is 6.43841 m.b.p. [1]. Pseudomonas aeruginosa is the size of the genome - 6.3 m.b.p., which contains 5570 genes on one chromosome [2]. Bacteria in addition to the main chromosome contain small extrachromosomal DNA which called - plasmids. Sizes of plasmids ranged from several thousand to hundreds of thousands of base pairs, and the number of copies per cell - from one to several hundred. Plasmids capable of autonomous (independent of the main chromosome) replication and is stably inherited in a number of cell generations. Many plasmids given host cell tangible selective advantage - resistance to antibiotics, heavy metals, the ability to degrade various xenobiotics, etc. Members of the genus Pseudomonas are most effective in combating various types of pollutants. They are essentially the "omnivorous." The cells of these organisms contain a hydroxylase and oxidoreductase, which can degrade a large number of molecules of hydrocarbons and aromatic compounds such as benzene, xylene, and toluene. Decomposition of aromatic acids can begin with non-oxidative decarboxylation, leading to the formation of phenols, which are then oxidized in the linear unsaturated dicarboxylic acid [3]. The genes encoding these enzymes are part of the plasmids [4]. САМ and NAH plasmids provide their own transport, inducing hybridization of bacterial cells, other plasmids can be transferred only if the bacteria have other plasmids, to ensure cross. [5]. Chakrabarti [6] after a successful crossing was a strain containing the plasmid XYL and NAH, as well as a hybrid plasmid obtained by recombination of parts of the CAM and OCT plasmids (by themselves they are not compatible, then there can not exist as a separate plasmid in a bacterial cell). This strain is able to grow rapidly in the crude oil, as it metabolizes hydrocarbons are much more active than any of the strains containing only one plasmid. The strain can be especially useful in treatment ponds for wastewater, where we can control the temperature and other external factors. In the study of strains of Pseudomonas aeruginosa Belhaj. et al [7] reported the presence of the genetic information of some alkane-monooxygenase. Many strains have been found genes similar to genes alkB1 and alkB2, that found in Pseudomonas putida. This suggests that in degradation of long chain alkanes in Pseudomonas aeruginosa are responsible for at least two alkane-hydroxylase complexes. Some strains were detected third hydroxylase complex encoded by the gene alkB [8]. These strains were able to utilize a wider range of alkanes (С12С22 и С6-С22), and were also able to degrade toxic insoluble alkanes. In the oxidation of alkanes involving membrane-bound monooxygenase encoded by the gene alkB and electron-transport system consisting of two rubredoxin and NADHdependent reductase rubredoxin encoded by the genes respectively rubA, rubA2 and rubB. [9]. These genes are part of the plasmid pUCP20 or strain of Pseudomonas aeruginosa plasmid in the OST strain Pseudomonas putida GPo1. [10]. Vetrova and others [11] reported a higher hydrocarbon activity in strain Pseudomonas chlororaphis PCL1391, which contains a plasmid pBS216. The most well described by way of degradation of alkanes encoded OCT plasmid of the strain Pseudomonas putida Gpo1 (formerly Pseudomonas oleovorans). Membrane-bound monooxygenase, soluble rubredoxin and rubredoxin reductase serve to convert alkanes to alcohols. The alcohol is then oxidized to aldehyde and the corresponding acid in the β-oxidation and the citric acid cycle. It shown, that the stimulation of oil biodegradation in the presence of Pseudomonas aeruginosabiosurfactant. [12]. Among the greatest positive effect of biosurfactant shows ramnolipids. Thus, because the ability to degradation of various pollutants depends on the enzyme composition of microorganisms, the use of computer analysis of nucleotide sequences of their genomes to evaluate and identify among them the most efficient degraders. References: 1. Paulsen I.T., Press C.M., Ravel J. Complite genome sequence of the plant commensal P. fluorescens Pf-5 // Nature Biotechnology. - 2005. - V.23. - P. 873878. 2. Stover C.K., Pham X.Q., Erwin A.L., Mizoguchi S.D., et al. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen // Nature. -2000. - V. 406. - №6799. -P. 959-964. 3. Abalos A., Vinas M., Manresa M.A., Solanas A.M. Enhanced Biodegradation of Casablanca Crude Oil by A Microbial Consortium in Presence of a Rhamnolipid Produced by Pseudomonas Aeruginosa AT10 // Biodegradation. - 2004. -V. 15. - № 4. -P. 249–260. 4. Dennis J., Zylstra G. Complete sequence and genetic organization of pDTG1, the 83 kilobase naphthalene degradation plasmid from Pseudomonas putida strain NCIB 9816-4 // J. Mol. Biol. - 2004. - V. 341. - P. 753–768. 5. Chakrabarty A.M. Plasmids in Pseudomonas // Annu. Rev. Genet. - 1976. № 10. - Р. 7-30. 6. Belhaj A., Desnoues N., Elmerich C. Alkane biodegradation in Pseudomonas aeruginosa strains isolated from a polluted zone: identification of alkB and alkB-related genes // Res. Microbiol. - 2002. -№ 153. - Р. 339-344. 7. Hagelueken G., Wiehlmann L., Adams T. M., Kolmar H., Heinz D.W., Tüummler B., Schubert W.-D. Crystal structure of the electron transfer complex rubredoxin–rubredoxin reductase of Pseudomonas aeruginosa // Proc Natl Acad Sci U S A. - 2007. – V.104. - № 30. –P. 12276–12281. 8. Smits T., Witholt B., van Beilen J. Functional characterization of genes involved in alkane oxidation by Pseudomonas aeruginosa // Antonie van Leeuwenhoek. -2003. - № 84. – P.193–200. 9. Marin M., Yuste L., Rojo F. Differential Expression of the Components of the Two Alkane Hydroxylases from Pseudomonas aeruginosa // J Bacteriol. 2003. -V.185. - № 10. - Р. 3232–3237. 10. van Beilen J., Neuenschwander M., Smits T.H., Roth C., Balada S.B., Witholt B. Rubredoxins involved in alkane oxidation // J Bacteriol. - 2002. №184. –P.1722–1732. 11. Vetrova A.A., Nechaev I.A., Ignatova A.A. and ext. Effect of catabolic plasmids on physiological parameters of bacteria Pseudomonas and the efficiency of biodegradation of oil / / Microbiology. - 2007. -V.76. - № 3. - P. 354-360. 12. Balba M., Al-Shayji Y., Al-Awadhi N., Yateem A. Isolation and characterization of biosurfactant-producing bacteria from oil-contaminated soil // Soil and Sediment Contamination. – 2002. - №11. – P.41–55.