Acid-Base Solutions Simulation Worksheet
advertisement

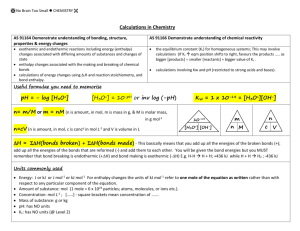
Acid-Base Solutions Simulation Introduction 1. Take a few minutes to play with the sim (http://phet.colorado.edu/en/simulation/acid-base-solutions). Check out both the Introduction and Custom Solution tabs. Explore what factors affect pH. List the factors you found that affect pH. Investigating Concentration Changes 2. a. Create a strong acid solution in the “Custom Solution” tab. b. Draw bar graphs for Initial and Equilibrium concentrations. Hints: No calculator needed – try the ‘Equilibrium Concentration’ view. Don’t forget to label your graphs! c. What equilibrium concentrations are affected by changing the initial concentration? 3. a. Create a weak acid solution in the “Custom Solution” tab. b. Draw bar graphs for Initial and Equilibrium concentrations. Hints: No calculator needed – try the ‘Equilibrium Concentration’ view. Don’t forget to label your graphs! c. What equilibrium concentrations are affected by changing the initial concentration? 4. Are your results for the strong and weak acid in questions 2 and 3 consistent with the definition of strong and weak acids? Investigating the Effects of Acid Strength and Concentration 5. a. What does the ‘strength’ slider (in the sim) effect? b. What does the term ‘strength’ mean? (In your own words) 6. How does strength affect the pH of acids? 7. How does initial concentration affect the pH of acids? 8. a. Is it possible for a solution of weak acid and a solution of strong acid to have the same pH? Design and carry out an experiment using the simulation to answer this question. What are your results? b. What was your strategy for testing whether a solution of strong acid and a solution of weak acid can have the same pH? pH Scale Simulation 1) You need to get familiar with adjusting the amount of liquid in the beaker. Choose water from the pull down menu. Fill and drain the water using the faucet knobs above and below the beaker. Try to get 0.5 L of water in the beaker. Using the slide arrow, adjust the flow. Play for a bit! Be sure to click the box shown. 2) Using the pull-down menu, change the contents of the beaker to “water”. What is the pH? ________ Record and compare (concentration mol/L) H3O+/OH- ratio. 3) Fill the beaker with drain cleaner. What is the pH? ________ Record and compare (concentration mol/L) H3O+/OH- ratio. 4) Fill the beaker with soda. What is the pH? ________ Record and compare (concentration mol/L) H3O+/OH- ratio. 5) Using “custom liquid”, adjust the pH level to 6.00. How does the H 3O+/OH- ratio differ from a pH of 7? Record and compare (concentration mol/L) H3O+/OH- ratio. 6) Again, using “custom liquid, adjust the pH level to 8.00. How does the H3O+/OH- ratio differ from a pH of 7? Record and compare (concentration mol/L) H3O+/OH- ratio. 7) Alright…let’s mix some acid items with water to see what happens to the H 3O+/OH- ratio! 8) Fill the beaker with coffee (pH 5.0). Drain out half, and replace that amount with water. What is the new pH? ________ Record (concentration mol/L) H3O+/OH- ratio. 9) OK, feeling nauseous? Fill er’ up with Barf!(pH 2.0) Drain out half, and replace it with H 2O! What is the new pH?________ Record (concentration mol/L) H3O+/OH- ratio. 10) Let’s get back to “basics”. LOL. Fill up with hand soap, drain half, and replace with water. What is the new pH? ________ Record (concentration mol/L) H3O+/OH- ratio. 11) What do you notice about the H3O+/OH- ratio when you add water to either an acid or a base? Hmmm….refer to how the mol/L changes. ____________________________________________________________________________________________________________ ____________________________________________________________________________________________________________ 12) Calculate: Add anything to the beaker. Using your scientific calculator, find the negative logarithm (-log) of H3O+ mol/L. This should match the pH value given. 13) Calculate: Add anything to the beaker. Using your scientific calculator, find the negative logarithm (-log) of OH- mol/L. This should match the pOH value given. 14) What is the pH of the solution to the left?