Acids and Bases - Calculations
advertisement

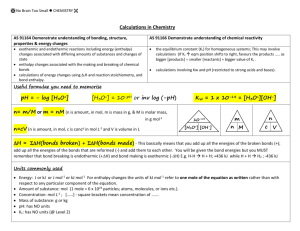
ACID-BASE CALCULATIONS (LIVE) 02 JUNE 2015 Section A: Summary Notes Acids and Bases - Calculations Neutralisation Reaction Neutralisation is the reaction between an acid and a base. The end point is reached when the acid and base are chemically equivalent. An indicator is used during neutralisation reactions to show the end point. An indicator is an organic compound which turns a specific colour in an acid or base and at a specific pH. Titration is the name given to process of performing a neutralisation reaction. Concentration The unit of concentration is mol.dm -3 , which is also written as M (stated as molar) A concentrated acid or base contains a large quantity of solute (acid or base) per volume of solution. A dilute acid or base contains a small quantity of solute (acid or base) per volume of solution. Calculations When a solution is diluted, the number of moles of the original solution stays the same even though the volume of the solution changes. The following equation is used. c1 = concentration of solution 1 (mol.dm -3) 𝑐1 𝑉1 = 𝑐2 𝑉2 c2 = concentration of solution 2 (mol.dm -3) V1 = volume of solution 1 (dm 3) V2 = volume of solution 2 (dm 3) For a titration reaction the following equation is used. na = number of moles of acid (mol) nb = number of moles of base (mol) 𝑛𝑎 𝑐𝑎 𝑉𝑎 = 𝑛𝑏 𝑐𝑏 𝑉𝑏 ca = concentration of acid (mol.dm -3) cb = concentration of base (mol.dm -3 Va = volume of acid (dm3) Vb = volume of base (dm3) pH calculations The pH of a solution is an indication of the acidity or alkalinity of a solution. It is the negative logarithm of the hydronium ion concentration in a solution. 𝑝𝐻 = = log[𝐻 + ] [H+] = concentration of H+ or H3O+ ions If the pH is known, [H+] is found by: [𝐻 + ] = 10−𝑝𝐻 Water undergoes auto-ionisation or auto-protolysis, in which a proton is transferred from one water molecule to another. This can be shown in the following equation: 𝐇𝟐 𝐎(𝓵) + 𝐇𝟐 𝐎(𝓵) ⇌ 𝐇𝟑 𝐎+ (𝐚𝐪) + 𝐎𝐇 − (𝐚𝐪) From this equation we get the equilibrium constant for this reaction, which is known as the dissociation constant for water: Kw = [H3O+][OH-] At 25o C, K w = [H3 O+ ][OH − ] = 1 × 10−14 , which means in a neutral solution that [H3O+] = [OH-] = 1 x 10-7 mol.dm-3 Therefore in aqueous solutions (at 25oC): [H3O+][OH-] = 1 x 10-14 Hydrolysis When an acid and a base react together one of the products formed is a salt. The salt will be neutral if a strong base and strong acid are reacted together. However, if the acid and base are not of comparable strengths then the salt formed will either be acidic or basic, depending on how it dissolves in water. Hydrolysis is the ability of the ions to react with the water molecules, thus altering the pH. Salt in water Example Salt of strong acid and strong base NaCl Salt of strong acid and weak base Salt of weak acid and strong base (HCl& NaOH) pH in aqueous solution pH = 7 NH4Cl pH < 7 (HCl& NH3) (acidic) CH3COONa pH > 7 (CH3COOH & NaOH) (basic) Example: Consider the salt NH4Cl. This salt is made from the reaction between NH3 (weak base) and HCl (strong acid). The Cl- ions will not react with the water molecules in the solution. But the 𝑁𝐻4+ ions will react with water according to the following equation: 𝑁𝐻4+ + 𝐻2 𝑂 ⇌ 𝑁𝐻3 + 𝐻3 𝑂+ Thus an excess of H3O+ ions are created and the solution will be acidic. Multiple Choice Questions Question 1 Which ONE of the following is a CORRECT description for a 0,1 mol.dm -3 hydrochloric acid solution? A. B. C. D. Dilute strong acid Dilute weak acid Concentrated weak acid Concentrated strong acid Question 2 Which ONE of the following represents the products formed during the hydrolysis of ammonium chloride? A. B. C. D. NH3(aq) and H3O+(aq) NH4+(aq) and Cl- (aq) HCl(aq) and OH-(aq) Cl-(aq) and H3O+(aq) Question 3 Consider the following reaction equilibrium: 𝑁𝐻3 (𝑔) + 𝐻2 𝑂(ℓ) ⇌ 𝑁𝐻4+ (𝑎𝑞) + 𝑂𝐻 − (𝑎𝑞) The two Bronsted-Lowry bases in the reaction equation are: A. B. C. D. NH3 and H2O NH4+ and OHH2O and NH4+ NH3 and OH- Question 4 Water undergoes auto-ionisation. During this process… A. B. C. D. a proton is transferred from one water molecule to another water molecules act as proton donors only water molecules act as proton acceptors only the pH of water will decrease Question 5 A small quantity of concentrated hydrochloric acid is gradually added to 1 dm 3 of distilled water at 25oC. After testing the resultant solution, it is found that the value of Kw, [H3O+] and [OH-] in mol.dm-3 are: A. Kw = 10-14 [H3O+] < 10-7 [OH-] > 10-7 B. Kw< 10-14 [H3O+] < 10-7 [OH-] < 10-7 C. Kw = 10-14 [H3O+] > 10-7 [OH-] < 10-7 D. Kw = 10-14 [H3O+] = 10-7 [OH-] = 10-7 Section B: Practice Questions Question 1 (Taken from Eastern Cape Paper 2 HG November2000) In an acid-base reaction, 500 cm 3 of a solution of sodium hydroxide is completely neutralised by 680 cm3 of a solution of sulphuric acid. The equation for the reaction is 𝐻2 𝑆𝑂4 (𝑎𝑞) + 2𝑁𝑎𝑂𝐻(𝑎𝑞) ⟶ 𝑁𝑎2 𝑆𝑂4 (𝑎𝑞) + 2𝐻2 𝑂(ℓ) The pH of the base solution before any acid is added is 13,80 at 25oC. 1.1. Show by calculation that the concentration of the NaOH solution will be 0,631 mol.dm-3. (4) 1.2. What is the difference between a strong base and a concentrated base? (2) 1.3. Calculate the mass of salt used to prepare the base solution. (4) 1.4. Calculate the number of moles of H3O+(aq) effectively used in the neutralisation process. (4) [14] Question 2 (Taken from Northern Cape Paper 2 HG November 2000) When 500 cm3 diluted hydrochloric acid of concentration 0,25 mol.dm -3 is added to 500 cm 3 of sodium hydroxide, the temperature of the solution rises and the pH changes to 2.3. 2.1. Classify this reaction as exothermic or endothermic. 2.2. Calculate 2.2.1. 2.2.2. the final concentration of the H+ ions the initial concentration of the sodium hydroxide solution. (1) (3) (8) Question 3 (Taken from Northern Province Paper 2 HG November 2000) A 20 cm3 solution of oxalic acid (COOH)2 is titrated against a 0,25 mol.dm -3 solution of NaOH of which the volume was 28 cm3. The unbalanced equation for the reaction is: (𝐶𝑂𝑂𝐻)2 (𝑎𝑞) + 𝑁𝑎𝑂𝐻(𝑎𝑞) ⟶ (𝐶𝑂𝑂𝑁𝑎)2 (𝑎𝑞) + 𝐻2 𝑂(ℓ) 3.1. 3.2. 3.3. Balance the equation. Calculate the concentration of the oxalic acid. The salt, sodium oxalate (COONa)2 reacts with water. What name is given to this reaction? Will the final solution for this reaction be acidic, basic or neutral? Give a reason for your answer. 3.4. (2) (4) (1) (4) Question 4 (Taken from KwaZulu Natal Paper 2 HG November 2000) A 0,5 dm3 solution is made up by dissolving 4,0 g of sodium hydroxide in water. 4.1. 4.2. Calculate the pH of this solution. (8) 20 cm3 of the above solution is neutralised by adding 40 cm 3 of dilute sulphuric acid solution. Calculate the concentration of the dilute sulphuric acid. (5) The dilute sulphuric acid solution in 4.2 was prepared by adding 10 cm 3 of concentrated acid to 490 cm3 of distilled water. Calculate the concentration of the concentrated sulphuric acid solution. (4) 4.3. Section C: Solutions Multiple Choice Questions 1. 2. 3. 4. 5. A A D A C Practice Questions Question 1 1.1. [𝐻 + ] = 10−𝑝𝐻 𝐾𝑤 = [𝐻 + ][𝑂𝐻 − ] = 10−14 = 10−13,80 (1,585 × 10−14 )[𝑂𝐻 − ] = 10−14 = 1,585 × 10−14 ∴ [𝑂𝐻 − ] = 0,631 [𝑁𝑎𝑂𝐻] = [𝑂𝐻 − ] = 0,631 𝑚𝑜𝑙. 𝑑𝑚−3 1.2. A strong base is a base that has a high percentage ionisation in water A concentrated base contains a large number of moles of base per unit volume of solution. 1.3. (4) M(NaOH) = 23 + 16 + 1 = 40 g.mol-1 𝑐= (2) 𝑚 𝑀𝑉 0,631 = 𝑚 (40)(0,5) ∴ 𝑚 = (0,631)(40)(0,5) = 12,62 𝑔 (4) 1.4. 𝑐(𝑁𝑎𝑂𝐻) = 0,631 = 𝑛 𝑉 𝑛 0,5 𝑛 = (0,631)(0,5) = 0,316 𝑚𝑜𝑙 0,316 mol H3O+is needed to neutralise 0,316 mol NaOH (4) Question 2 2.1. Exothermic 2.2. [𝐻 + ] = 10−𝑝𝐻 (1) = 10−2,3 = 5,01 × 10−3 𝑚𝑜𝑙. 𝑑𝑚−3 2.3. (3) Initial n(𝐻𝐶ℓ) 𝑛 𝑐= 𝑉 𝑛 0,25 = 0,5 Final n(𝐻𝐶ℓ) 𝑛 𝑐= 𝑉 𝑛 = (0,25)(0,5) 𝑛 = (5,01 × 10−3 )(1) = 0,125 𝑚𝑜𝑙 = 5,01 × 10−3 𝑚𝑜𝑙 5,01 × 10−3 = 𝑛 (0,5 + 0,5) 𝑛(𝐻𝐶ℓ) 𝑛𝑒𝑢𝑡𝑟𝑎𝑙𝑖𝑠𝑒𝑑 = 0,125 − 5,01 × 10−3 = 0,12 𝑚𝑜𝑙 𝐻𝐶ℓ + 𝑁𝑎𝑂𝐻 ⟶ 𝑁𝑎𝐶ℓ + 𝐻2 𝑂 ∴ 0,12 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 𝑟𝑒𝑎𝑐𝑡𝑠 𝑤𝑖𝑡ℎ 0,12 𝑚𝑜𝑙 𝐻𝐶ℓ 𝑐(𝑁𝑎𝑂𝐻) = 𝑛 𝑉 = 0,12 0,5 = 0,24 𝑚𝑜𝑙. 𝑑𝑚−3 (8) Question 3 3.1. (𝐶𝑂𝑂𝐻)2 (𝑎𝑞) + 2𝑁𝑎𝑂𝐻(𝑎𝑞) ⟶ (𝐶𝑂𝑂𝑁𝑎)2 (𝑎𝑞) + 2𝐻2 𝑂(ℓ) 3.2. 𝑛𝑎 𝑐𝑎 𝑉𝑎 = 𝑛𝑏 𝑐𝑏 𝑉𝑏 (2) (𝑐𝑎 )(0,02) 1 = (0,25)(0,028) 2 ∴ 𝑐𝑎 = (0,25)(0,028) (2)(0,02) = 0,175 𝑚𝑜𝑙. 𝑑𝑚−3 3.3. Hydrolysis (4) (1) 3.4. Basic (𝐶𝑂𝑂𝑁𝑎)2 → 𝐻2 𝑂 (𝐶𝑂𝑂− )2 + 2𝑁𝑎+ (𝐶𝑂𝑂− )2 + 2𝐻2 𝑂 ⟶ (𝐶𝑂𝑂𝐻)2 + 2𝑂𝐻 − The OH- formed during hydrolysis causes the solution to become basic (4) Question 4 4.1. M(NaOH) = 23 + 16 + 1 𝑐= = 40 g.mol-1 𝑚 𝑀𝑉 = 4 (40)(0,5) = 0,2 𝑚𝑜𝑙. 𝑑𝑚−3 [OH-] = [NaOH] = 0,2 mol.dm-3 Kw = [H+][OH-] 10-14 = [H+](0,2) [H+] = 5 x 10-14 mol.dm-3 pH = -log [H+] = -log (5 x 10-14) = 13,3 4.2. (8) H2SO4 + 2NaOH Na2SO4 + 2H2O 𝑛𝑎 𝑐𝑎 𝑉𝑎 = 𝑛𝑏 𝑐𝑏 𝑉𝑏 (𝑐𝑎 )(0,04) 1 = (0,2)(0,02) 2 ∴ 𝑐𝑎 = (0,2)(0,02) (2)(0,04) = 0,05 𝑚𝑜𝑙. 𝑑𝑚−3 4.3. (5) 𝑐1 𝑉1 = 𝑐2 𝑉2 (0,05)(0,5) = 𝑐2 (0,01) 𝑐2 = 2,5 𝑚𝑜𝑙. 𝑑𝑚3 (4)