SpectraPart2
advertisement

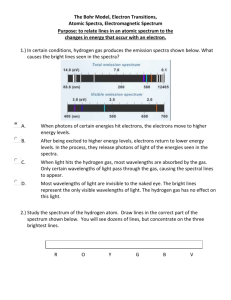
Astronomy Rough Notes – Bohr Model of Atom, Lines in Spectra BRING: Curtain Variac Clear, single filament light bulb Tennis balls Box/chair to stand on DISCLAIMER: These notes do NOT cover everything you need to know. You may need to look up some item or concept online or in a text. Test questions are not exact copies of the OBJECTIVES but if you know the OBJECTIVES thoroughly, you should do well on the exams. HANDOUTS: None OTHER RESOURCES: http://youtu.be/sVev5RsKXog (Parts 1 and 2) OBJECTIVES Describe the two major characteristics of black body radiation. Draw the intensity vs wavelength graph for a black body. What happens to that graph if the temperature of the body increases? If you turn up the temperature of a light bulb, what did you notice about the peak wavelength and about the overall radiation. Draw two graphs of intensity vs wavelength for the low temperature bulb and the high temperature bulb. Identify the three basic particles in an atom. Know whether their charge is positive or negative or neutral. Know their approximate relative masses (not the mass itself, just how they compare). Know where each is located in the atom. Describe or sketch a simple model of the atom (the Bohr model) including permitted orbits. Given a model of the atom, show which jumps correspond to emission spectra and which to absorption spectra. Given a model of the atom showing several energy levels, identify which photon comes from which electron transition. (See the tutorial.) What information can astronomers obtain from the spectrum of a star, galaxy or gas cloud? What did Annie Cannon contribute to the study of spectra? Cecilia Payne? State the two chief components of stars. What percent is each? Define and differentiate between the following: atom, element, molecule MATERIAL: Black body radiation Think of filament of light bulb 1. Billions and billions of atoms all vibrating at slightly different frequencies, emitting slightly different λ 2. A few long λ, a few short λ but most λ near the middle. 3. Graph Intensity vs λ I n t e n s i t y λpeak Light bulb demo As T rises: Color redder to bluer Brighter So as T rises: More overall radiation (intensity rises) Shorter peak λ ( λpeak ) For more on black body radiation, see http://www.uwgb.edu/dutchs/CosmosNotes/spectra.htm (Nice picture) Or http://www.spacegrant.montana.edu/msiproject/light.html Or http://www.nrao.edu/index.php/learn/radioastronomy/radiowaves Or http://www.uwgb.edu/dutchs/CosmosNotes/spectra.htm For an interactive simulation, see http://phet.colorado.edu/en/simulation/blackbody-spectrum Tutorial will be emailed to you. Be sure to work it. SPECTRAL LINES OF SUN Show spectral lines of sun See http://coolcosmos.ipac.caltech.edu/cosmic_classroom/cosmic_reference/images/solarspectra.jpg Absorption hot dense photosphere with gas around Note mix of lines/mix of elements SPECTRA OF OTHER STARS Each stripe of light is the spectrum of one star. Note that each spectrum tapers off at the ends like the black body graphs above. Not e the different lines in each spectrum for different stars. From: Sky Publishing Corp, Cambridge Mass. WHY SPECTRAL LINES Why lines in spectra? Why different lines? Physics in late 1800s Show stellar spectra/line patterns O stars are hot, M stars are cool Note how hot O stars show more in the shorter λ, cooler M stars show more in the longer λ Annie Cannon at Harvard University was legendary at classifying stars. She looked at over 100 000 star spectra and classified them by their relative temperatures. See video “Ring of Truth” Part 6 of 6 “Doubt” First 12 minutes Not available for free on the web. I have a copy in my office. Structure of the atom - Bohr model and connection to spectra http://www.colorado.edu/physics/2000/quantumzone/bohr.html and http://csep10.phys.utk.edu/astr162/lect/light/bohr.html Bohr model Electrons in atoms can have only certain “orbits” or energies and not others. Model the atom with electrons in orbit around the nucleus. Electrons can jump up or down between energy levels but can never be in between. Electrons can jump down by emitting energy Electron orbits Nucleus containing protons (+charge) and neutrons (neutral) Electrons can jump up if they absorb energy When an electron jumps down, it emits all its energy at once in a bundle called a photon. The bigger the jump, the higher the energy of the photon. When an electron absorbs a photon of just the right energy, it jumps up. Example – A model atom is show below. 2 3 1 Question: Of the electron jumps shown, which jumps emit photons and which absorb photons? Emit: Absorb: Question: Suppose you are told that a blue and a green photon are emitted and a red photon is absorbed. Which jump corresponds with which? Blue photon emitted Green photon emitted Red photon absorbed Example tennis ball demo Tutorial will be sent Video: “Ring of Truth” again Begin ~13:30 Quantum ladder A word about the video The video shows these orbits as a quantum ladder like the one shown below. The ground state is the lowest energy the electron can have. State 2 State 1 Ground state --------------------------------------------------------------------------------------------------------------------Which photon has the highest energy emitted from the electron transitions shown? ---------------------------------------------------------------------------------------------------------------------- 1 2 3 Green, red, and violet photons are emitted from the atom whose quantum ladder is shown on the left. Which color comes from which transition? What color lines are in the spectrum of this atom? Continue video through Cecilia’s thesis to ~26:00 Also see http://www.colorado.edu/physics/2000/quantumzone/ Recipe of stars and universe All that “glows” is 90% H and 10% He See last page for summary of spectra and electron transitions. Homework Make a flashcard for each objective. Read. Work the tutorial on Blackbody radiation that was emailed. Work the tutorial on “Light and Atoms” that was emailed. Revised 5 January 2016 Astronomy – Brief Review of Spectra and Connection to the Atom Observation: 3 Types Continuous Emission Absorption Description Continuous display of Only certain present Certain missing Source Hot, dense like hot metal Excited (hot) gas Radiation from hot, dense source passes through cool gas Each element has a distinct set of emission and/or absorption lines. ******************************************************************************* Model: Atoms exist only with discrete energies. Atoms of each element have a distinct set of energy levels. Energy levels can be represented as a quantum ladder. Example State 2 State 1 Energy An electron (represented by a dot ) can be in state 2 or in state 1 or in the ground state but it cannot be in between. Ground state ******************************************************************************* Explains Emission Spectra: This electron transition down (small jump down) emits a low energy photon (like red). Many of these jumps emit many “red” photons that show up as one red line in the spectrum. This electron transition down (big jump down) emits a high energy photon (like violet). Many of these jumps down emit “violet” photons that show up as one violet line in the spectrum. This electron transition down emits an intermediate energy photon (like green). Many of these jumps down emit many “green” photons that show up as one green line in the spectrum. ******************************************************************************** Explains Absorption Spectra: This electron transition up (small jump up) absorbs a low energy photon (like red). Many of these jumps up This electron transition up (big jump show up as one missing red line in the spectrum. up) absorbs a high energy photon (like violet). Many of these jumps up absorb “violet” photons that show up as one missing violet line in the spectrum. This electron transition up absorbs an intermediate energy photon (like green). Many of these jumps up absorb many “green” photons that show up as one missing green line in the spectrum. Example of absorption spectrum Hot, dense surface Emits photons of all (continuous spectrum) All photons H gas cloud Absorbs certain photons All photons minus those absorbed by H Astronomer sees absorption spectrum (continuous but missing H )