Qualitative Analysis – Identification of Ions in Solution by Solubilities
advertisement

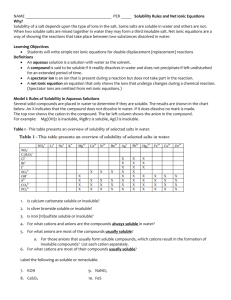
Qualitative Analysis – Identification of Ions in Solution by Solubilities Safety: Solutions in this experiment are fairly dilute and present only small hazards when brought in contact with the skin. Thorough rinsing with water is appropriate for treating contact. Specific hazards are listed at the end of this handout. As always, contact with the eyes should be avoided at all costs, so safety glasses should be worn at all times in the lab. Qualitative analysis: If faced with the problem of identifying ions in a solution, one frequently attempts to explore their solubility properties. For instance, a solution that forms a precipitate when a BaCl2 solution is added to it may contain SO42- ion; or a solution that forms precipitates when an NaCl solution is added to it may contain Ag+. This is because BaSO4 and AgCl are known to be insoluble, Ba2+ and SO42-, or Ag+ and Cl- can not exist together in solution – they fall out of solution and form precipitates. Your objectives today will be to identify the products of combinations of two solutions of ionic materials and to write the net ionic equations for any reactions. You may want to review the material on solubility and net ionic equations in your text book, p 149,150. A generic molecular equation for the combination of solutions of two binary ionic materials (i.e. materials composed of one type of anion and one type of cation) is: C1A1 + C2A2 C1A2 + C2A1 , where the C’s represent cations and the A’s represent anions. The only possible reaction involves the rearrangement of the ions, and that limits the number of possible products to two since two cations can not combine, nor can two anions. This is sometimes referred to as a double displacement reaction. Any ionic material which is soluble in water is assumed to break apart into ions in solution. The reactant solutions in today’s lab can be viewed as having solutes that are totally dissociated into ions. Consequently, if two of these solutions are mixed and no change is observed, one concludes that the ions are still dissociated so no reaction must have occurred. In such a case there is no net ionic equation. Evidence of a reaction when two solutions are mixed includes color changes, the formation of precipitates, the evolution of heat, or bubbling. For the sake of this discussion, we will focus on the formation of precipitates, i.e. one of the possible products is insoluble and precipitates from solution. In these cases one is faced with the task of identifying which of the two possible products is the solid precipitate. C1A1 (aq) + C2A2 (aq) C1A2 (s?) + C2A1 (s?) Additional information is needed. For example, a precipitate forms when CaCl2(aq) and AgNO3(aq) are mixed. Without additional information, you can’t determine if the precipitate is either AgCl(s) or Ca(NO3)2(s). But if you also know, or observe, that no reaction occurs when CaCl2(aq) and KNO3(aq) solutions are mixed, you can eliminate the possibility that Ca(NO3)2(s) is formed. Consequently, the precipitate in the original mixture must be AgCl(s). Once the precipitate is identified the generic net ionic equation can be written: C2+(aq) + A1-(aq) C2A1(s). Precipitates form when two ions which compose an insoluble product are brought together. There is not a well-defined criterion, however, for assigning substances the title of “soluble” or “insoluble”. Some folks use a molarity criterion for the saturated solution such as 0.01M, or a mass per volume criteria such as 5g/100mL(1). In each case, substances that can make solutions of higher concentration than the criterion are referred to as “soluble”, and substances that are unable to make solutions as concentrated as the criteria are referred to as “insoluble”. Not only are there different criteria in use , but even with one criteria, substances are not simply either “soluble” or “insoluble”; those terms simply represent the extremes of a continuum. There are many substances whose saturated solutions have concentrations close to the criteria and hence would be more accurately identified as “slightly soluble” or “sparingly soluble”. In today’s lab you will be combining pairs of solutions that are as low as 0.10M, so your resulting solutions will be as lower than 0.1M. These solutions are several times more concentrated than some common solubility criteria, so they may show precipitation in some situations in which the product may be identified as “soluble”. Fortunately, we will side-step any uncertainty in interpreting the solubility rules by empirically generating our own rules. Those observations will determine your solubility rules for the sake of today’s lab. It will be useful to compare your observations with the solubility rules listed in your textbook. Flame Tests: Tests like the ones you will be doing today can be designed to determine just about any ion in solution. Some ions however are virtually impossible to identify in solution using these precipitation tools, but they can be determined with other simple tools. The Flame Test is one such tool. In a Flame Test, a bit of solution is heated rapidly in a flame, and the ions emit light of distinct colors – different colors for different elements. Today you will carry out a Flame Test to distinguish Na+ from K+. The Na+ gives rise to a yellow-orange flame, while the K+ gives rise to a purplish flame. To analyze small portions of solution, one wets a nonreactive wire with the solution of interest then puts the wire in a flame. The wire must not create a strong emission itself, so not all wires are appropriate. Wires made from Platinum or an alloy of Nickel and Chromium are commonly used. The wires must also be reproducibly clean, so they are rinsed in HCl prior to each use. Procedure: - Obtain the set of 10 solutions: HCl, Cu(NO3)2, BaCl2, KCl, Na2CO3, NaCl, Al2(SO4)3, NaOH, Na2SO4, and AgNO3. For purposes of describing the different solution combinations, these solutions will be referred to as #1, #2,…,#10. You will need to keep good clear notes in your lab notebook and on your lab bench to keep things straight. There are plenty of labels available to keep solutions clearly identified. You will work with a lab partner in this experiment, but you should share solutions with your other neighbors so you can make better use of the disposable pipettes and not waste the solutions. - Use the disposable pipettes to put 5 drops of each solution in the wells of your wellplate as in the figure below. You should mix the contents of the cells by swirling the entire well-plate. - Record any changes you see. Specify precipitation, color of solution, color of precipitate, bubbling. In some instances you will see brief changes immediately, and in some instances you may need to wait a minute or two for the changes to develop. [To notice changes, you must know the appearance of each solution before it is combined with another solution.]. - Wash the well-plates before and after use. - Be careful not to contaminate any cells with solution from other cells – make sure any stir rods used for mixing are clean and dry each time, and use each pipette in only one solution. - Write net ionic equations for the combinations that produce a reaction. Compare your results with a neighbor. If you find a discrepancy, fix it. Flame Test: - Take a piece of NiChrome wire. Swish it around in the concentrated HCl for a few seconds, then hold it in the flame. Repeat this until there is a consistent color (probably a bit yellowish) when in the flame. Next, put the end of the wire in the solution of interest then immediately put it in the hottest part of the Bunsen burner flame. Note the color of the flame. Perform a flame test on each of the 10 solutions. You should be able to distinguish Na+ from K+ using this technique, and you may need it to identify your unknown. - Obtain an unknown, and record its ID. Combine 5 drops of the unknown solution with 5 drops of each of the known solutions in your well plate. Perform a flame test if necessary. Deduce the identity of the unknown. [It is one of the original 10.] - Dispose of the waste solutions in the bottle on the back bench. All the solutions may be combined together in the waste container. Before leaving, clean your well-plate, making sure it does not have any residues left in it. Be sure your lab report includes… … an Abstract indicating the objective of the lab and a list of the materials you determined were insoluble. Indicate the identity of your unknown and the key observations that led you to make that conclusion. … a table indicating the identity of the 10 solutions and the pairs that showed a reaction. Characterize the reaction products it by color or amount of precipitate. Note any that do not follow the solubility rules listed in the text book. … the net ionic equations for all the observed reactions. … the identity of your unknown solution, and explain your thought process in making that conclusion. … an answer to the question: Based on your observations, would you expect to be able to combine solutions of BaSO4 and a Na2CO3 and form a precipitate ? If you have problems with the question, indicate why. -------------------------------------------------------------------------------------------------Specific Material Hazards: Cu(NO3)2 - toxic and an irritant Al2(SO4)3 - irritant AgNO3 - toxic and corrosive, stains skin NaOH - toxic and corrosive BaCl2 - toxic and an irritant HCl - irritant toxic and corrosive --------------------------------------------------------------------------------------------------References: 1 Heimerzheim, C. J., J. Chem. Ed. 1941, 18, 377