Light Emission Spectroscopy Lab Worksheet
advertisement

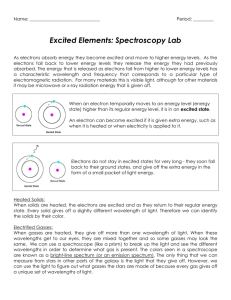
Name _________________________ Partner’s Name(s) _________________________ Excited Elements - Light Emission Spectroscopy Introduction: As electrons absorb energy they become excited and move to higher energy levels. As the electrons fall back to lower energy levels they release the energy they absorbed in set amounts called quanta. The energy that is released as electrons fall from higher to lower energy levels has a characteristic wavelength and frequency that corresponds to a particular type of electromagnetic radiation. For example, when electrons fall from a higher energy level down to the 2nd energy level, the wavelength and frequency of the energy produced correspond to that of visible light. Electrons of atoms can be excited in various ways including heat, electricity and friction. For example, a solution of sodium chloride placed on a platinum wire and held in a flame emits a bright, yellow light that is characteristic of the metallic element sodium. Another method of spectrum analysis involves the application of high voltage across a gas-filled glass tube. Gases under low pressure and excited by an electrical discharge give off light in characteristic wavelengths. The emitted light is passed through a spectroscope, which breaks light into its components for analysis. A gas viewed through a spectroscope, such as the one shown in Figure 1, forms a series of bright lines known as a bright-line or emission spectrum. Since each element produces a unique bright-line spectrum or pattern, spectroscopy is a valuable branch of science for determining what elements are present. The composition of stars and other objects in outer space is determined using this technique. Unlike gases, heated solids produce a continuous spectrum. A gas is identified by comparing the wavelengths of its emission (bright line) spectrum to the spectrum produced by a known gas. In this experiment, you will use a spectroscope to determine the bright-line spectra characteristic of different elements. Purpose: To observe the characteristic bright line spectra produced by applying high voltage across a sample of a gas at very low pressure and to determine the identity of an unknown gas. Materials/Equipment: High voltage power supplies Diffraction grating glasses Spectroscopes 40-watt incandescent bulb/socket Thermal mitt Fluorescent bulb/socket Colored Pencils (ROY G. BIV) Label Cards for each spectral tube Spectral tubes: Helium Neon Oxygen Mercury Nitrogen Hydrogen Unknown Safety Considerations: DO NOT TOUCH the spectrum-tube power supply or spectrum tubes when power is applied. Several thousand volts exist at the power supply and spectrum tubes. ASIM Spectrophotometry: Excited Elements Revised: 6/06 p. 1 Name _________________________ Partner’s Name(s) _________________________ Procedure: 1. Obtain a spectroscope and look through it at an incandescent light bulb. The spectrum should appear when the slit in the spectroscope is pointed just off center of the glowing filament. Practice moving the spectroscope until you see a bright, clear image. 2. Darken the room but leave enough background lighting to illuminate the spectroscope scales. Point the spectroscope away from any exposed window, since daylight will affect the observed gas spectrum. 3. Helium or hydrogen is a good first choice among the spectral tubes set up around the room. Adjust the spectroscope until the brightest image is oriented on your scale. Record in Table 1 the five brightest lines of the observed spectrum. Some of the spectrum tubes produce light so dim that you must be very close to them to get good observations of the spectral lines. 4. Repeat for each of the other spectrum tubes. Figure 1: Look through the spectroscope at the emitted light of the spectrum tubes. ASIM Spectrophotometry: Excited Elements Revised: 6/06 p. 2 Name _________________________ Partner’s Name(s) _________________________ Data: Draw in lines at the proper locations and of the correct width and intensity to reflect what you have observed. On the line at the right of each scale, write the name of the element you are observing. Sketch of line spectrum O2 V I B G Y O R Y O R Y O R Y O R Y O R Y O R Sketch of line spectrum N2 V I B G Sketch of line spectrum Ne V I B G Sketch of line spectrum Hg V I B G Sketch of line spectrum H2 V I B G Sketch of line spectrum He V I ASIM Spectrophotometry: Excited Elements Revised: 6/06 B G p. 3 Name _________________________ Partner’s Name(s) _________________________ Questions: 1. How do electrons respond when energy is added? 2. When materials are heated, or receive energy, their electrons ______________________. 3. The color of the light is characteristic of the ____________________________________. 4. Every element has its own pattern of emitted light…a . 5. We can look at this pattern through a . 6. Light passes through a slit, then through the grating and separates into . 7. This is called a/an . 8. This method is used by astronomers to determine the composition of the . 9. What produces the color of fireworks? 10. Explain how it is possible for colored “neon lights” to be changed to reflect the colors of a specific product (advertisement signs) or to enhance the appearance of a business sign. 11. Are all the lines of the spectral patterns of the same intensity? Explain. 12. Are they always in the same order: ROYGBIV? Explain. 13. Why are the lines different colors? Applications: 1. Describe the uses of a spectroscope in the science of astronomy. 2. How can spectra be used in chemical analyses? ASIM Spectrophotometry: Excited Elements Revised: 6/06 p. 4