Supplementary Tables Table 5 Ocular (study eye) and non
advertisement
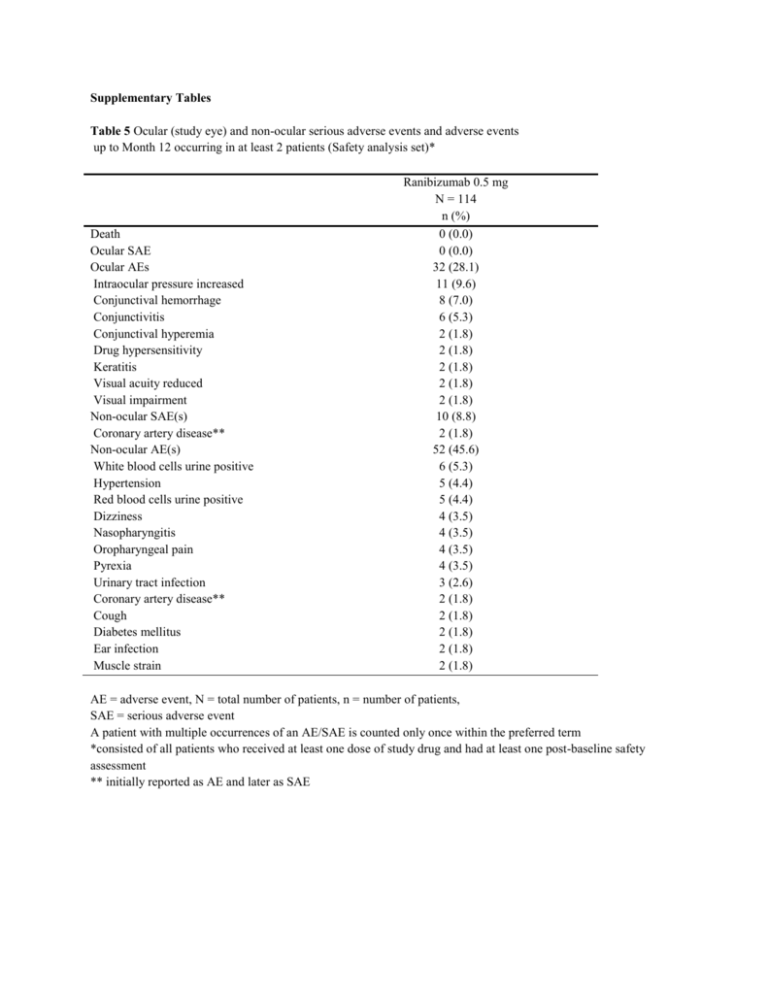
Supplementary Tables Table 5 Ocular (study eye) and non-ocular serious adverse events and adverse events up to Month 12 occurring in at least 2 patients (Safety analysis set)* Death Ocular SAE Ocular AEs Intraocular pressure increased Conjunctival hemorrhage Conjunctivitis Conjunctival hyperemia Drug hypersensitivity Keratitis Visual acuity reduced Visual impairment Non-ocular SAE(s) Coronary artery disease** Non-ocular AE(s) White blood cells urine positive Hypertension Red blood cells urine positive Dizziness Nasopharyngitis Oropharyngeal pain Pyrexia Urinary tract infection Coronary artery disease** Cough Diabetes mellitus Ear infection Muscle strain Ranibizumab 0.5 mg N = 114 n (%) 0 (0.0) 0 (0.0) 32 (28.1) 11 (9.6) 8 (7.0) 6 (5.3) 2 (1.8) 2 (1.8) 2 (1.8) 2 (1.8) 2 (1.8) 10 (8.8) 2 (1.8) 52 (45.6) 6 (5.3) 5 (4.4) 5 (4.4) 4 (3.5) 4 (3.5) 4 (3.5) 4 (3.5) 3 (2.6) 2 (1.8) 2 (1.8) 2 (1.8) 2 (1.8) 2 (1.8) AE = adverse event, N = total number of patients, n = number of patients, SAE = serious adverse event A patient with multiple occurrences of an AE/SAE is counted only once within the preferred term *consisted of all patients who received at least one dose of study drug and had at least one post-baseline safety assessment ** initially reported as AE and later as SAE Table 6 Ocular (study eye) adverse events up to Month 12 (Safety analysis set)* Ranibizumab 0.5 mg N = 114 n (%) 32 (28.1) 11 (9.6) 8 (7.0 ) 6 (5.3) 2 (1.8) 2 (1.8) 2 (1.8) 2 (1.8) 2 (1.8) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) Total Intraocular pressure increased Conjunctival hemorrhage Conjunctivitis Conjunctival hyperemia Drug hypersensitivity Visual impairment Keratitis Visual acuity reduced Blepharitis Cataract Exophthalmos Eyelid edema Retinal hemorrhage Subretinal fibrosis Vitreous floaters Vitreous hemorrhage Vitreous opacities N = total number of patients, n = number of patients A patient with multiple occurrences of an adverse event is counted only once within the preferred term *consisted of all patients who received at least one dose of study drug and had at least one post-baseline safety assessment Table 7 Non-ocular adverse events up to Month 12 (Safety analysis set)* Total White blood cells urine positive Red blood cells urine positive Hypertension Nasopharyngitis Dizziness Pyrexia Oropharyngeal pain Urinary tract infection Ear infection Muscle strain Cough Coronary artery disease** Diabetes mellitus Blood glucose increased Blood uric acid increased Glucose urine present Protein urine present Urine analysis abnormal White blood cell count increased Folliculitis Gastroenteritis Pneumonia Cerebral artery stenosis Cerebral thrombosis Polyneuropathy Sciatica Transient ischemic attack Face edema Non-cardiac chest pain Edema peripheral Fracture Injury Muscle injury Skin laceration Ranibizumab 0.5 mg N = 114 n (%) 52 (45.6) 6 (5.3) 5 (4.4) 5 (4.4) 4 (3.5) 4 (3.5) 4 (3.5) 4 (3.5) 3 (2.6) 2 (1.8) 2 (1.8) 2 (1.8) 2 (1.8) 2 (1.8) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) Table 7 (Continued) Non-ocular adverse events up to Month 12 (Safety analysis set)* Ranibizumab 0.5 mg N = 114 n (%) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) Subdural hemotoma Chronic obstructive pulmonary disease Hypotension Vena cava thrombosis Extrasystoles Hyperlipidemia Arthralgia Bone formation increased Pain in jaw Spinal osteoarthritis Diarrhoea Enteritis Hemorrhoids Proctocolitis Cholangitis acute Cholecystitis Lung adenocarcinoma metastatic Multiple myeloma Agitation Hematuria N = total number of patients, n = number of patients A patient with multiple occurrences of an adverse event is counted only once within the preferred term *consisted of all patients who received at least one dose of study drug and had at least one post-baseline safety assessment ** initially reported as AE and later as SAE Table 8 Non-ocular serious adverse events up to Month 12 (Safety analysis set)* Ranibizumab 0.5 mg N = 114 n (%) 10 (8.8) 2 (1.8) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) 1 (0.9) Total Coronary artery disease Cerebral artery stenosis Cerebral thrombosis Cholangitis acute Cholecystitis Chronic obstructive pulmonary disease Hypertension Lung adenocarcinoma metastatic Multiple myeloma Pneumonia Subdural hematoma Transient ischemic attack N = total number of patients, n = number of patients A patient with multiple occurrences of a serious adverse event is counted only once within the preferred term *consisted of all patients who received at least one dose of study drug and had at least one post-baseline safety assessment