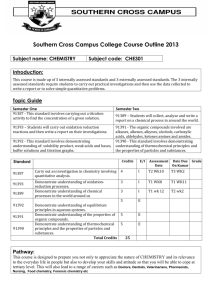
10 - chemistrysuzhou
advertisement

AS Chemistry Section 1: Energetics Unit 2: Physical Chemistry Part 1: Enthalpy change Task 1 Use your knowledge of IGCSE Chemistry to complete the notes below. Enthalpy change, H Energy changes are a characteristic feature of chemical reactions. Many chemical reactions give out energy, and a few take energy in. A reaction that gives out energy and heats the surroundings is described as _______________. A reaction that takes in energy and cools the surroundings is _______________. During an exothermic reaction the chemical reactants are losing energy. This energy is used to heat the surroundings – e.g. the _______________, the _______________, the _______________. The products end up with less energy than the reactants had – but the surroundings end up with more, and get hotter. We measure the energy transferred to and from the surroundings as enthalpy change, H. Enthalpy changes can be shown on an enthalpy level diagram, also called an energy level diagram. N.B. we cannot measure the enthalpy (or heat content), H, of a substance. What we can do is measure the change in enthalpy when a reaction occurs. Task 2 Draw and label enthalpy level diagrams for (i) the reaction of methane with oxygen and (ii) the thermal decomposition of calcium carbonate. Show the progress of the reactions (from reactants to products) along the X-axis and enthalpy along the Yaxis. On each diagram mark the enthalpy change, state whether its value is positive or negative, and hence describe the reaction as exothermic or endothermic. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry How much? As your study of chemistry becomes more advanced, you will find that you need to put numbers to the features of chemical reactions – to make them quantitative. We measure the energy changes associated with chemical reactions by measuring enthalpy changes. For an exothermic reaction ΔH is negative. This is because, from the point of view of the chemical reactants, energy has been lost to the surroundings. Conversely, for an endothermic reaction ΔH is positive - energy has been gained from the surroundings. The units of ΔH Enthalpy changes are measured in kilojoules per mole. E.g. for the reaction of methane with oxygen, we write: CH4(g) + 2O2(g) CO2(g) + 2H2O(l) ΔH = -890 kJ mol-1 This means that for every mole of methane that reacts in this way, 890 kJ of energy are transferred to heat the surroundings. If we used 2 moles of methane, we should get 2 x 890 = 1780 kJ of energy transferred. This assumes that all the methane is converted into products, and that none is left unburned. Standard conditions Like most physical and chemical quantities, ΔH varies according to the conditions. In particular, ΔH is affected by temperature, pressure and concentration of solutions. So we choose certain standard conditions to refer to. We use: a specified temperature (usually 298K) a standard pressure of 100kPa a standard concentration of 1 mol dm-3. The symbol ΔHθ is used for standard enthalpy changes. Elements and compounds are said to be in their standard state if they are in their normal, stable state at 298K and 100kPa. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Task 3 What are the standard states of the following substances? Pentane Iodine Mercury Ethanoic acid Sulphur dioxide Water Sulphur Carbon Measuring enthalpy changes Many enthalpy changes can be measured quite simply in the laboratory. We usually do it by arranging for the energy involved in the reaction to be transferred to or from water. If it is an exothermic reaction, the water gets hotter; if it is endothermic, the water gets cooler. If we measure the temperature change of the water, and if we know its mass and specific heating capacity, we can work out how much energy was transferred to or from the water during the chemical reaction. To do this, we need to us the relationship: q = m x c x ΔT where: q is the heat energy transferred, c is the specific heating capacity of water (4.18 kJ K-1kg-1 or 4.18 J K1 -1 g ) m is the mass of the water, and ΔT is the change in temperature of the water. The heat energy, q, can then be used to calculate an enthalpy change. Task 4 a) In an experiment, 0.16g of methanol is used up when a spirit burner heats 100g of water from 17oC to 25oC. Calculate the molar enthalpy change of combustion of methanol. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Explain why the value obtained is much less than the value given in a data book. b) In an insulated container, 50cm3 of 2.0M HCl at 293K were added to 50cm3 of 2.0M NaOH also at 293K. After reaction, the temperature of the mixture rose to 307K. Calculate the molar enthalpy change for this reaction. Different kinds of enthalpy change The following kinds of enthalpy change are particularly important and are given special names: Standard enthalpy change of combustion, ΔHθc, 298 is the enthalpy change when 1 mole of a substance is burned completely in oxygen under standard conditions (100kPa and 298K), all reactants and products being in their standard states. At this temperature water is usually taken to be a liquid. The enthalpy change is linked to an equation with state symbols. Task 5 Write an equation to represent the enthalpy of combustion of butane. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Standard enthalpy change of formation, ΔHθf, 298 is the enthalpy change when 1 mole of a compound is formed from its elements under standard conditions (100kPa and 298K), all reactants and products being in their standard states. At this temperature water is usually taken to be a liquid. Task 6 Give a value for the enthalpy of formation of aluminium. Task 7 Write an equation to represent the enthalpy of formation of methane. There is a problem with the reaction shown above. It doesn’t actually occur under normal conditions. So how did anyone manage to measure the value of ΔHθf, 298? It has to be done indirectly, making use of quantities that can be measured and incorporating these into an enthalpy cycle. We will look at this next lesson. References A-level Chemistry pages 71-79 Chemistry in Context pages 146-150 Learning Objectives Candidates should be able to: Explain that some chemical reactions are accompanied by energy changes, principally in the form of heat energy. Construct (and interpret) a reaction pathway diagram, in terms of the enthalpy change of the reaction. Calculate enthalpy changes from appropriate experimental results, including the use of the relationship: q=mcT. Explain and use the terms enthalpy change of reaction and standard conditions, with particular reference to formation and combustion. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Section 1: Energetics Part 2: Enthalpy cycles The first law of thermodynamics Energy can neither be created nor destroyed but it can be converted from one form to another. Hess’ Law The enthalpy change of a reaction depends only on the initial and final state of the reaction and is independent of the route by which the reaction may occur. There is both a direct and an indirect way to turn carbon (graphite) and hydrogen into methane. We can’t measure the enthalpy change for the direct route. The indirect route goes via CO2 and H20 and involves enthalpy changes which we can measure. Worked example Use the following standard enthalpy changes of combustion to calculate the standard enthalpy of formation of methane. Hc(C) Hc(H2) Hc(CH4) Cambridge A-level Centre = = = -393 kJmol-1 -286 kJmol-1 -890 kJmol-1 AS Chemistry Unit 2: Physical Chemistry We can express this relationship mathematically: H = (Hc reactants) - (Hc products) You may also be asked to derive the Hc for a substance given Hf values. This time the equation to be used is: H = (Hf products) - (Hf reactants) Task 1 Complete Qs 1-4 on the worksheet ‘Hess’ Law’. Please lay out your answers neatly showing each stage in your calculation. Other standard enthalpy changes Standard enthalpy change of hydration, ΔHθhyd, 298 is the enthalpy change when 1 mole of a gaseous ion dissolves in water to give an infinitely dilute solution. Task 2 Can you write an equation for ΔHθhyd (Na+) Enthalpies of hydration are always negative, i.e. hydration is exothermic and energy is given out. Standard enthalpy change of solution, ΔHθsol, 298 is the enthalpy change when 1 mole of a solute dissolves in a solvent to give an infinitely dilute solution. Task 3 Can you write an equation for ΔHθsol (NaCl) Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Standard enthalpy change of neutralisation, ΔHθneut, 298 is the enthalpy change when 1 mole of H+ ions from an acid is completely neutralised by an alkali to give one mole of water. Standard enthalpy change of atomisation, ΔHθat, 298 is the enthalpy change when 1 mole of gaseous atoms is formed from one mole of the element in its standard state. Task 4 Can you write an equation to represent: (i) the standard enthalpy change of atomisation of sodium, (ii) the standard enthalpy change of atomisation of chlorine. References A-level Chemistry pages 81-84 Chemistry in Context pages 150-153 Learning Objectives Candidates should be able to apply Hess’ Law to construct simple energy cycles, and carry out calculations involving such cycles and relevant energy terms, with particular reference to determining enthalpy changes that cannot be found by direct experiment, e.g. an enthalpy change of formation from enthalpy changes of combustion. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Section 1: Energetics Part 3: Bond enthalpies All chemical reactions involve breaking and making chemical bonds. Bonds break in the reactants and new bonds form in the products. The energy changes associated with chemical reactions come from the energy changes when bonds are broken and made. A chemical bond is basically an electrical attraction between atoms or ions. When you break a bond, you have to do work in order to overcome these attractive forces. To break the bond completely, you need (theoretically) to separate the atoms or ions so they are an infinite distance apart. The quantity of energy needed to break a particular bond in a molecule is called the bond dissociation enthalpy (H diss), or bond enthalpy for short. It refers to the enthalpy change when one mole of bonds of the same type are broken in gaseous molecules under standard conditions. Bond enthalpies are very useful, because they tell you how strong bonds are. The stronger a bond, the more difficult it is to break, and the higher its bond enthalpy. E.g H–H (g) H(g) + H(g) The bond enthalpy of the H-H bond is +436kJ mol-1. Notice that this is a positive H value, because breaking a bond is an endothermic process – it needs energy. When you make a new bond, you get energy given out. All hydrogen molecules are identical and there is no difference between the atoms in the molecules. Now consider methane. This contains four equal C-H bonds. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry But as soon as we start to pull apart the methane molecule, the remaining C-H bonds exist in a different environment. . Since the energy needed to break the bonds depends on the environment of the bond, the eventual figure for the enthalpy change for the dissociation of C-H bonds in methane will be an average of four values. The bond enthalpy values quoted in data books are mean bond enthalpy values averaged over a wide range of different compounds. Bond enthalpy values are very difficult to measure directly. They are usually calculated from other enthalpy values. Another way to calculate the enthalpy change of a reaction is to consider which bonds are broken and which are made. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Worked example Let’s consider the combustion of ethanol in more detail. Bond Number broken Number formed Average Molar Bond Enthalpy kJ mol-1 C-C 1 0 +347 C-H 5 0 +413 C-O 1 0 +358 O-H 1 6 +464 O=O 3 0 +498 C=O 0 4 +805 Here bond enthalpies are defined endothermically. Bond breaking: Total endothermic value = (+347 x 1) + (+413 x 5) + (+358 x 1) + (+464 x 1) + (+498 x 3) = +4728 kJ Bond making: Total exothermic value = (-464 x 6) + (-805 x 4) = -6004 kJ Sum total of bond breaking and bond making: Hc = +4728 + - 6004 = -1276 kJ mol-1 Task 1 (a) State what is meant by the term mean bond enthalpy. ................................................................................................................... ................................................................................................................... ................................................................................................................... Cambridge A-level Centre (2) AS Chemistry (b) Unit 2: Physical Chemistry Ethanal has the structure H H C O C H H Gaseous ethanal burns as shown by the equation CH3CHO(g) + 2½O2(g) → 2H2O(g) + 2CO2(g) Use the mean bond enthalpy data given below to answer the following questions. (i) Bond Mean bond kJmol-1 C—H +413 C—C +347 C=O +736 O=O +498 O—H +464 enthalpy / Calculate the enthalpy change which occurs when all the bonds in the reactants shown in the above equation are broken. (ii) Calculate the enthalpy change which occurs when all the bonds in the products shown in the above equation are formed. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry (iii) Hence, calculate the enthalpy change for the complete combustion of ethanal as shown in the equation above. (5) (Total 7 marks) Task 2 Use bond enthalpies to calculate a value for the enthalpy change of the reaction between nitrogen gas and hydrogen gas to form ammonia. The bond enthalpy of NN is +945 kJmol-1,of H-H is +436 kJmol-1 and that of N-H is +391 kJmol-1. References A-level Chemistry pages 81-84 Chemistry in Context pages 150-153 Learning Objectives Candidates should be able to apply Hess’ Law to construct simple energy cycles, and carry out calculations involving such cycles and relevant energy terms, with particular reference to average bond energies. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Section 2: Kinetics Part 1: Collision Theory and Activation Energy Different chemical reactions go at different rates. Task 1 Can you name some reactions which, at standard temperature and pressure, fit the timescale shown? Seconds Minutes Hours Days Weeks Months Years Decades Centuries Millennia The meaning ‘rate of reaction’ When we talk about the rate of something we mean the rate at which some quantity changes. Speed, for example, is the rate of change of distance. Whenever you are measuring a rate you need to be clear about the units you are using. Speed is often measured in metres per second. The rate of a reaction is found by measuring the amount in moles of a reactant which is used up, or the amount of product produced, in a given time. The units are often mol dm -3 s-1. The study of rates of reactions is referred to as chemical kinetics. There are many reasons why chemists study reaction rates, for example to: Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry improve the rate of production of a chemical; gain an insight into the mechanism of a reaction (A2); help understand the processes going on in our bodies or in the environment. Task 2 There are a number of factors which can affect the rate of a chemical reaction. Can you list them below? Collision Theory We can explain the effect of these factors using Collision Theory. The basic idea is that reactions occur when the particles of reactants collide, provided they collide with a certain minimum amount of kinetic energy (and in the correct orientation). Activation Energy Activation energy is the minimum energy required before a reaction can occur. You can show this on an energy level diagram for the reaction. For a simple over-all exothermic reaction, it looks like this: If the particles collide with less energy than the activation energy, nothing important happens. They bounce apart. You can think of the activation energy as a Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry barrier to the reaction. Only those collisions which have energies equal to or greater than the activation energy result in a reaction. Any chemical reaction results in the breaking of some bonds (needing energy) and the making of new ones (releasing energy). Obviously some bonds have to be broken before new ones can be made. Activation energy is involved in breaking some of the original bonds. Task 3 The enthalpy diagrams A-D represent four different reactions. All the diagrams are drawn to the same scale. A B Enthalp y Enthalp y Reactants Products Reactants Products Progress of Reaction Progress of Reaction C D Which of the diagrams: a) Represent exothermic reactions? b) Represent endothermic reactions? c) Shows the largest activation enthalpy? d) Shows the smallest activation enthalpy? e) Represents the most exothermic reaction. f) Represents the most endothermic reaction? Enthalp y Enthalp y Products Reactants Products Progress of Reaction Reactants Progress of Reaction The Maxwell-Boltzmann Distribution Because of the key role of activation energy in deciding whether a collision will result in a reaction, it would obviously be useful to know what sort of proportion of the particles present have high enough energies to react when they collide. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry In any system, the particles present will have a very wide range of energies. For gases, this can be shown on a graph called the Maxwell-Boltzmann Distribution which is a plot of the number of particles having a particular amount of energy. You should also note: there are no particles with zero energy there is no maximum energy for particles. The area under the curve is a measure of the total number of particles present. The Maxwell-Boltzmann Distribution and activation energy Remember that for a reaction to happen, particles must collide with energies equal to or greater than the activation energy for the reaction. We can mark the activation energy on the Maxwell-Boltzmann distribution (see diagram below). Notice that the large majority of the particles don't have enough energy to react when they collide. To enable them to react we either have to change the shape of the curve, or move the activation energy further to the left. We will look at this later. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry References A-level Chemistry pages 166-170 Chemistry in Context pages 352-355, 366-367 Learning Objectives Candidates should be able to: Explain and use the terms rate of reaction and activation energy. Show understanding, including reference to the Boltzmann distribution, of what is meant by the term activation energy. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Section 2: Kinetics Part 2: Effect of temperature Task 1 The collision theory assumes that the rate of a reaction depends on: A. How often the reactant particles collide with one another, and B. The proportion of reactant molecules that have enough energy to react once they have collided. Which out of A and B, explains each of the following observations? Observation Letter Reactions in solution go faster at higher concentration. Solids react faster with liquids or gases when their surface area is greater. Catalysts increase the rate of reactions. Increasing the temperature increases the rate of a reaction. Temperature effects As you increase the temperature the rate of reaction increases. As a rough approximation, for many reactions happening at around room temperature, the rate of reaction doubles for every 10°C rise in temperature. You have to be careful not to take this too literally. It doesn't apply to all reactions. Even where it is approximately true, it may be that the rate doubles every 9°C or 11°C or whatever. The number of degrees needed to double the rate will also change gradually as the temperature increases. Examples Some reactions are virtually instantaneous - for example, a precipitation reaction involving the coming together of ions in solution to make an insoluble solid, or the reaction between hydrogen ions from an acid and hydroxide ions from an alkali in solution. So heating one of these won't make any noticeable difference to the rate Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry of the reaction. Almost any other reaction you care to name will happen faster if you heat it - either in the lab, or in industry. The explanation Increasing the collision frequency Particles can only react when they collide. If you heat a substance, the particles move faster and so collide more frequently. That will speed up the rate of reaction. That seems a fairly straightforward explanation until you look at the numbers! It turns out that the frequency of two-particle collisions in gases is proportional to the square root of the Kelvin temperature. If you increase the temperature from 293 K to 303 K (20°C to 30°C), you will increase the collision frequency by a factor of (see page 366 of Chemistry in Context): That's an increase of 1.7% for a 10° rise. The rate of reaction will probably have doubled for that increase in temperature - in other words, an increase of about 100%. The effect of increasing collision frequency on the rate of the reaction is very minor. The important effect is quite different . . . The key importance of activation energy We have seen that collisions only result in a reaction if the particles collide with enough energy to get the reaction started. This minimum energy required is called the activation energy for the reaction. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Only those particles represented by the area to the right of the activation energy will react when they collide. The great majority don't have enough energy, and will simply bounce apart. To speed up the reaction, you need to increase the number of the very energetic particles - those with energies equal to or greater than the activation energy. Increasing the temperature has exactly that effect - it changes the shape of the graph. In the next diagram, the graph labelled T is at the original temperature. The graph labelled T+t is at a higher temperature. If you now mark the position of the activation energy, you can see that although the curve hasn't moved very much overall, there has been such a large increase in the number of the very energetic particles that many more now collide with enough energy to react. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Remember that the area under a curve gives a count of the number of particles. On the last diagram, the area under the higher temperature curve to the right of the activation energy looks to have at least doubled - therefore at least doubling the rate of the reaction. Summary Increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions. It is only these collisions (possessing at least the activation energy for the reaction) which result in a reaction. Task 2 Can you complete the past paper question ‘Effect of Temperature’. References A-level Chemistry pages 168-170 Chemistry in Context pages 365-369 Learning Objectives Candidates should be able to: Explain qualitatively, in terms of both of the Boltzmann distribution and of collision frequency, the effect of temperature change on the rate of reaction. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Section 2: Kinetics Part 3: Catalysis The Effect of Catalysts on Reaction Rates What are catalysts? A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. When the reaction has finished, you would have exactly the same mass of catalyst as you had at the beginning. Task 1 The table below shows some examples of catalysed reactions which you have, or will, come across in your A-level course. Use the index in your textbook to find the catalyst which is commonly used. Reaction a. Decomposition of hydrogen peroxide b. Nitration of benzene c. Manufacture of ammonia by the Haber Process d. Conversion of SO2 into SO3 during the Contact Process to make sulphuric acid e. Hydrogenation of a C=C double bond Cambridge A-level Centre Catalyst AS Chemistry Unit 2: Physical Chemistry The explanation We have seen that particle collisions only result in a reaction if the particles collide with enough energy to get the reaction started. This minimum energy required is called the activation energy. You can mark the position of activation energy on a Maxwell-Boltzmann distribution to get a diagram like this: Only those particles represented by the area to the right of the activation energy will react when they collide. The great majority don't have enough energy, and will simply bounce apart. Catalysts and activation energy To increase the rate of a reaction you need to increase the number of successful collisions. One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. In other words, to move the activation energy on the graph like this: Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry A catalyst provides an alternative route for the reaction. This alternative route has a lower activation energy. Task 2 The diagram below shows an enthalpy level diagram for both a catalysed and uncatalysed reaction. Can you add appropriate labels to both curves and to each of the arrows? A word of caution! Be very careful if you are asked about this in an exam. The best way to say it is: "A catalyst provides an alternative route for the reaction with a lower activation energy." It does not "lower the activation energy of the reaction". There is a subtle difference between the two statements that is easily illustrated with a simple analogy. Suppose you have a mountain between two valleys so that the only way for people to get from one valley to the other is over the mountain. Only the most active people will manage to get from one valley to the other. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Now suppose a tunnel is cut through the mountain. Many more people will now manage to get from one valley to the other by this easier route. You could say that the tunnel route has a lower activation energy than going over the mountain. But you haven't lowered the mountain! The tunnel has provided an alternative route but hasn't lowered the original one. The original mountain is still there, and some people will still choose to climb it. In the chemistry case, if particles collide with enough energy they can still react in exactly the same way as if the catalyst wasn't there. It is simply that the majority of particles will react via the easier catalysed route. Homogeneous and heterogeneous catalysis Catalysts can be divided into two main types: heterogeneous and homogeneous. Heterogeneous catalysis: where the reactants and catalyst are in different physical states. This usually involves a mixture of gases or liquids reacting in the presence of a solid catalyst. Homogenous catalysis: where the reactants and catalyst are in the same physical state. enzyme-catalysed reactions in cells take place in aqueous solution and are examples of this type of catalysis. Task 3 For each of the reactions in task 1 above, decide whether the catalysis is homogeneous or heterogeneous. a. b. c. d. e. Enzymes Enzymes are proteins that act as biological catalysts. Without them the reactions that make life possible would be too slow for life to exist. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Task 4 Can you choose appropriate words to describe the properties of enzymes? Enzymes differ from inorganic catalysts because they are: __________ – catalyse only particular molecules (or classes of molecules). __________ – produce few side-products. __________ - they are affected by changes in pH and temperature. __________ - able to perform their task in a highly organized way. Example of the ‘lock-and-key’ mechanism of enzyme catalysis. References A-level Chemistry pages 170-172 Chemistry in Context pages 369 - 373 Learning Objectives Candidates should be able to: explain that, in the presence of a catalyst, a reaction has a different mechanism, i.e. one of lower activation energy, and interpret this catalytic effect in terms of the Boltzmann distribution. describe enzymes as biological catalysts (proteins) which may have specific activity Cambridge A-level Centre AS Chemistry Section 2: Kinetics Unit 2: Physical Chemistry Part 4: Concentration (or Pressure) The effect of concentration on reaction rates For many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction. (In a few cases, increasing the concentration of one of the reactants may have little noticeable effect on the rate. These cases are discussed at A2.) Don't assume that if you double the concentration of one of the reactants that you will double the rate of the reaction. It may happen like that but the relationship may well be more complicated. It depends on the mechanism of the reaction. Task 1 The examples given here all involve solutions. Changing the concentration of a gas is achieved by changing its pressure. In each case can you write a balanced equation for the reaction taking place and say how you could measure the rate of this reaction. Zinc and hydrochloric acid In the lab, zinc granules react fairly slowly with dilute hydrochloric acid, but much faster if the acid is concentrated. Equation: What could you measure? The catalytic decomposition of hydrogen peroxide Solid manganese(IV) oxide is often used as a catalyst in this reaction. Oxygen is given off much faster if the hydrogen peroxide is concentrated than if it is dilute. Equation: What could you measure? The reaction between sodium thiosulphate solution and hydrochloric acid Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry This is a reaction which is often used to explore the relationship between concentration and rate of reaction in introductory courses (like IGCSE). When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed. Equation: What could you measure? As the sodium thiosulphate solution is diluted more and more, the precipitate takes longer and longer to form. The explanation Cases where changing the concentration affects the rate of the reaction This is the common case, and is easily explained. The same argument applies whether the reaction involves collision between two different particles or two of the same particle. In order for any reaction to happen, those particles must first collide. This is true whether both particles are in solution, or whether one is in solution and the other a solid. Increasing the concentration of a reagent increases the number of particles in a given volume, and so increases the chance of productive collisions. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Task 2 Sketch a concentration-time graph for the reaction of zinc with hydrochloric acid. Mark where the reaction is fastest and where it is effectively over. Explain the shape of your graph. The Effect of Pressure on Reaction Rates Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. Changing the pressure on a reaction which involves only solids or liquids has no effect on the rate. An example In the manufacture of ammonia by the Haber Process, the rate of reaction between the hydrogen and the nitrogen is increased by the use of very high pressures. In fact, the main reason for using high pressures is to improve the percentage of ammonia in the equilibrium mixture, but there is a useful effect on rate of reaction as well. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry The explanation Increasing the pressure of a gas is exactly the same as increasing its concentration. If you have a given mass of gas, the way you increase its pressure is to squeeze it into a smaller volume. If you have the same mass in a smaller volume, then its concentration is higher. You can also show this relationship mathematically using the ideal gas equation: Rearranging this gives: Because "RT" is constant as long as the temperature is constant, this shows that the pressure is directly proportional to the concentration. If you double one, you will also double the other. The effect of increasing the pressure on the rate of reaction In order for any reaction to happen, those particles must first collide. This is true whether both particles are in the gas state, or whether one is a gas and the other a solid. If the pressure is higher, the chances of collision are greater. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Task 3 Can you find and correct the errors on the worksheet ‘Kinetics – find the errors’. References A-level Chemistry pages 166 -169 Chemistry in Context page 354 Learning Objectives Candidates should be able to: explain qualitatively, in terms of collisions, the effect of concentration changes on the rate of a reaction. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Section 3: Redox reactions Part 1: Definitions Consider the reaction below: Zn + CuO ZnO + Cu The Zn has gained oxygen, we say it has been oxidised. The copper oxide has lost oxygen, we say it has been reduced. Definitions Oxidation is gain of oxygen. Reduction is loss of oxygen. Because both reduction and oxidation are going on side-by-side, this is known as a redox reaction. A similar reaction occurs in the extraction of iron from its ore. Task 1 Can you write a balanced equation for the reduction of iron ore inside the blast furnace? Label clearly which substance has been oxidised and which has been reduced. Oxidising and reducing agents An oxidising agent is substance which oxidises something else. A reducing agent reduces something else. Task 2 On your diagram above label the oxidising and reducing agents. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Oxidation and reduction in terms of hydrogen transfer These are old definitions. However, you may find them useful in studying organic reactions. Definitions Oxidation is loss of hydrogen. Reduction is gain of hydrogen. Notice that these are exactly the opposite of the oxygen definitions. For example, ethanol can be oxidised to ethanal: Ethanal can also be reduced back to ethanol again. Oxidation and reduction in terms of electron transfer Let’s consider the reaction below: Zn + MgCl2 ZnCl2 + Mg This is analogous to our first reaction but doesn’t involve oxygen. We need a more general way to describe such processes. Definitions Oxidation is loss of electrons. Reduction is gain of electrons. It is essential that you remember these definitions. There is a very easy way to do this. As long as you remember that you are talking about electron transfer: Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Copper(II) chloride and magnesium chloride are both ionic. The metals obviously aren't. If you rewrite this as an ionic equation, it turns out that the chloride ions are spectator ions and you are left with: Task 3 In the reaction above which substance is the reducing agent and which is the oxidising agent. What has happened to each? Task 4 a) Insert electrons (e-) on the appropriate side of the following half-equations in order to balance and complete them, so that the electrical charges on both sides are equal. Such equations are known as electron half-equations. i. ii. iii. iv. v. K H2 O Cu+ Cr3+ K+ 2H+ O2Cu2+ Cr2+ b) For each completed half-equation describe the process as oxidation or reduction. Oxidation States (Oxidation numbers) Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Oxidation states simplify the whole process of working out what is being oxidised and what is being reduced in redox reactions. They also allow us to deal with redox processes which do not involve ions. Working out oxidation states You don't work out oxidation states by counting the numbers of electrons transferred. It would take far too long. Instead you learn some simple rules, and do some very simple sums! The oxidation state of an uncombined element is zero. The sum of the oxidation states of all the atoms or ions in a neutral compound is zero. The sum of the oxidation states of all the atoms in an ion is equal to the charge on the ion. The more electronegative element in a substance is given a negative oxidation state. The less electronegative one is given a positive oxidation state. Some elements almost always have the same oxidation states in their compounds: element usual oxidation state Group 1 metals always +1 Group 2 metals always +2 Oxygen usually -2 except in peroxides and F2O (see below) Hydrogen usually +1 except in metal hydrides where it is -1 (see below) Fluorine always -1 Cambridge A-level Centre exceptions AS Chemistry Chlorine Unit 2: Physical Chemistry usually -1 except in compounds with O or F (see below Chlorine in compounds with fluorine or oxygen There are so many different oxidation states that chlorine can have in these, that it is safer to simply remember that the chlorine doesn't have an oxidation state of -1 in them, and work out its actual oxidation state when you need it. You will find an example of this below. Task 5 Write down the oxidation states of the elements in the following examples. a. Ag+ h. CO2 b. Br2 i. PCl5 c. P4 j. Al2O3 d. H+ k. SF6 e. H- l. SO42- f. N3- m. NO3- g. MgCl2 n. PO43- References A-level Chemistry pages 94-95 Chemistry in Context pages 20-21 Learning Objectives Candidates should be able to: describe and explain redox processes in terms of electron transfer and/or of changes in oxidation number (oxidation state) Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Section 3: Redox reactions Part 2: Using oxidation states Naming compounds You will have come across names like iron(II) sulphate and iron(III) chloride. The (II) and (III) are the oxidation states of the iron in the two compounds: +2 and +3 respectively. That tells you that they contain Fe2+ and Fe3+ ions. This terminology can be extended to the negative ion. The sulphate ion is SO42-. The oxidation state of the sulphur is +6 (work it out!). The ion is more properly called the sulphate(VI) ion. A sulphite ion is SO32-. The oxidation state of the sulphur is +4 (work that out as well!). This ion is more properly called the sulphate(IV) ion. So FeSO4 is properly called iron(II) sulphate(VI), and FeSO3 is iron(II) sulphate(IV). In fact, because of the easy confusion between these names, the old names sulphate and sulphite are normally still used in introductory chemistry courses. Task 1 Use oxidation states to name the ions or compounds with the formulae: a. b. c. d. e. f. g. n. SnO SnO2 FeCl2 FeCl3 PbCl4 Cu2O Mn(OH)2 VO3- Cambridge A-level Centre h. i. j. k. l. m. NO2NO3SO32SO42MnO4CrO42- AS Chemistry Unit 2: Physical Chemistry Using oxidation states to identify what's been oxidised and what's been reduced. This is easily the most common use of oxidation states. Remember: Oxidation involves an increase in oxidation state Reduction involves a decrease in oxidation state Task 2 Some reactions of the halogens and halogen ions are shown below. They are all redox reactions. In each case state which element is oxidised and which is reduced, and give the oxidation states before and after the reaction. a. 2ClO3b. 2Brc. 8Id. I2 + + + 2Cl- 2H+ + + 3O2 H2SO4 8H+ + H2SO4 SO3- + H 2O Br2 4I2 2I- + + + SO2 + H 2S SO42- + + 2H2O 4H2O 2H+ Task 3 In each of the following examples, you have to decide whether the reaction involves redox, and if so what has been oxidised and what reduced. 1. The reaction between magnesium and hydrochloric acid or hydrogen chloride gas: 2. The reaction between sodium hydroxide and hydrochloric acid is: Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry 3. This is a sneaky one! The reaction between chlorine and cold dilute sodium hydroxide solution is: Example 3 shows a disproportionation reaction. A disproportionation reaction is one in which a single substance is both oxidised and reduced. Writing ionic equations for redox reactions Half-equations When magnesium reduces hot copper(II) oxide to copper, the ionic equation for the reaction is: You can split the ionic equation into two parts, and look at it from the point of view of the magnesium and of the copper(II) ions separately. This shows clearly that the magnesium has lost two electrons, and the copper(II) ions have gained them. These two equations are described as "half-equations" or "electron-halfequations" or "ionic-half-equations" or "half-reactions" - lots of variations all meaning exactly the same thing! Any redox reaction is made up of two half-equations: in one of them electrons are being lost (an oxidation process) and in the other one those electrons are being gained (a reduction process). Working out half-equations and using them to build ionic equations Example: The reaction between chlorine and iron(II) ions Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry In a reaction chlorine gas oxidises iron(II) ions to iron(III) ions. In the process, the chlorine is reduced to chloride ions. Write a balanced equation for this redox process. Process You start by writing down what you know for each of the half-reactions. In the chlorine case, you know that chlorine (as molecules) turns into chloride ions: Then balance the atoms: ALWAYS check that you have the existing atoms balanced before you do anything else. If you forget to do this, everything else that you do afterwards is a complete waste of time! Now you have to add things to the half-equation in order to make it balance completely. All you are allowed to add are: electrons water hydrogen ions (unless the reaction is being done under alkaline conditions - in which case, you can add hydroxide ions instead) In the chlorine case all that is wrong with the equation we've produced so far is that the charges don't balance. The left-hand side of the equation has no charge, but the right-hand side carries 2 negative charges. That's easily put right by adding two electrons to the left-hand side. The final version of the half-reaction is: Now you repeat this for the iron(II) ions. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Combine the half-reactions to make the ionic equation for the reaction What we've got at the moment is this: It is obvious that the iron reaction will have to happen twice for every chlorine molecule that reacts. Allow for that, and then add the two half-equations together. Check that everything balances - atoms and charges. It is very easy to make small mistakes, especially if you are trying to multiply and add up more complicated equations. Task 4 The first example was a simple bit of chemistry which you may well have come across. The technique works just as well for more complicated (and perhaps unfamiliar) chemistry. Try to write balanced equations for the reactions described below. a. The reaction between hydrogen peroxide and manganate(VII) ions Manganate(VII) ions, MnO4-, oxidise hydrogen peroxide, H2O2, to oxygen gas. The reaction is done with potassium manganate(VII) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry During the reaction, the manganate(VII) ions are reduced to manganese(II) ions. b. The oxidation of ethanol by acidified potassium dichromate(VI) This technique can be used just as well in examples involving organic chemicals. Potassium dichromate(VI) solution acidified with dilute sulphuric acid is used to oxidise ethanol, CH3CH2OH, to ethanoic acid, CH3COOH. The oxidising agent is the dichromate(VI) ion, Cr2O72-. This is reduced to chromium(III), Cr3+. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry References A-level Chemistry pages 94-95 Chemistry in Context pages 20-24 Learning Objectives Candidates should be able to: describe and explain redox processes in terms of electron transfer and/or of changes in oxidation number (oxidation state). Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Section 3: Redox reactions Part 3: Manufacturing chlorine Task 1 Salt (sodium chloride) forms transparent colourless crystals. Describe the bonding in sodium chloride crystals, give the formula of each particle and sketch part of the crystal structure. Explain why crystals of sodium chloride do not conduct electricity, but molten sodium chloride does. ........................................................................................................................................... ........................................................................................................................................... ........................................................................................................................................... Task 2 Use the information on pages 226-227 of ‘Chemistry in Context’ and pages 96 and 114 of ‘A-level Chemistry’, along with your own scientific knowledge to complete the notes below. Background chemistry Chlorine is manufactured by electrolysing __________ (a concentrated solution of sodium chloride). In fact, the electrolysis of sodium chloride solution can be Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry used to make three useful substances - chlorine, __________ __________and __________. The chemistry of the electrolysis process Sodium chloride solution contains the following ions: The hydrogen and hydroxide ions come from the equilibrium: At any one time, the concentration of hydrogen ions or hydroxide ions will be very small - the __________ _____ __________ lies well to the left-hand side. At the anode The __________, chloride and hydroxide, get attracted towards the positively charged __________. Under standard conditions it is actually slightly easier to liberate hydroxide ions (to give oxygen) than chloride ions (to give chlorine), but there are far, far more chloride ions arriving at the anode than hydroxide ions. The major reaction at the anode is therefore: __________ chloride ions each give up an __________ to the anode, and the atoms produced combine to give __________ __________. At the cathode Sodium ions and hydrogen ions (from the __________) are attracted to the __________. It is much easier for a hydrogen ion to pick up an electron than for a sodium ion. So this reaction happens: As the hydrogen ions are converted into hydrogen gas, the water equilibrium tips to the __________ to replace them. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry The net effect of this is that there is a build up of sodium ions and these newly-produced hydroxide ions around the cathode. In other words, __________ __________ solution is being formed around the cathode. The need to keep all the products separate If __________ comes into contact with hydrogen, it produces a mixture which will explode __________ on exposure to sunlight or heat. Hydrogen chloride gas would be produced. Obviously, the two gases need to be kept apart. Chlorine also reacts with __________ __________ solution to produce a mixture of sodium chloride (NaCl) and sodium chlorate(I) (__________) - also known as sodium hypochlorite. This mixture is commonly sold as __________. Therefore, if you are trying to manufacture chlorine and sodium hydroxide rather than bleach, you have to keep the chlorine and sodium hydroxide apart as well. The diaphragm The diaphragm is made of a porous mixture of __________ and polymers. The solution can seep through it from the anode compartment into the cathode side. Notice that there is a __________ level of liquid on the anode side. That makes sure that the flow of liquid is always from __________ to __________ - preventing any of the sodium hydroxide solution formed finding its way back to where chlorine is being produced. The diaphragm cell is designed so that all the products are kept separate. This is shown in the diagram overpage. Major uses of the products chlorine: .............................................................................................................................. hydrogen: ........................................................................................................................ sodium hydroxide............................................................................................................... Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry The diaphragm cell References A-level Chemistry pages 96 and 114 Chemistry in Context pages 226-227 Learning Objectives Candidates should be able to explain, including the electrode reactions, the industrial process of the electrolysis of brine, using a diaphragm cell. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Section 3: Redox reactions Part 4: Aluminium Task Read the information below on the extraction of aluminium from its ore. Use this information to answer the questions which follow. Extracting aluminium from bauxite Aluminium is mined as the ore bauxite, aluminium oxide-2-water, which contains silicon(IV) oxide and iron(III) oxide as impurities. After purification we are left with pure, anhydrous aluminium oxide. Aluminium is too high in the electrochemical series (reactivity series) to extract it from its ore using carbon reduction. The temperatures needed are too high to be economical. Instead, it is extracted by electrolysis. The aluminium oxide is first dissolved in molten cryolite, Na3AlF6 - another aluminium compound. The aluminium oxide has too high a melting point to electrolyse on its own. The electrolysis cell The diagram shows a very simplified version of an electrolysis cell. Although the carbon lining of the cell is labelled as the cathode, the effective cathode is mainly the molten aluminium that forms on the bottom of the cell. Molten aluminium is syphoned out of the cell from time to time, and new aluminium oxide added at the top. The cell operates at a low voltage of about 5 - 6 volts, but at huge currents of 100,000 amps or more. The heating effect of these large currents keeps the cell at a temperature of about 950°C. The electrode reactions Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry These are very complicated - in fact they may not yet be fully understood. For chemistry purposes at this level, they are always simplified. This is the simplification: Aluminium is released at the cathode. Aluminium ions are reduced by gaining 3 electrons. Oxygen is produced initially at the anode. However, at the temperature of the cell, the carbon anodes burn in this oxygen to give carbon dioxide and carbon monoxide. Continual replacement of the anodes is a major expense. Properties Aluminium is usually alloyed with other elements such as silicon, copper or magnesium. Pure aluminium isn't very strong, and alloying adds to its strength. Aluminium is especially useful because it has a low density; is strong when alloyed; is a good conductor of electricity; has a good appearance; resists corrosion because of the strong thin layer of aluminium oxide on its surface. This layer can be strengthened further by anodising the aluminium. Some economic and environmental considerations The production of aluminium uses a great deal of electricity. This is because you have to add a lot of electrons (because of the high charge on the ion) to produce a small mass of aluminium (because of its low relative atomic mass). Hydroelectric power is the most economical form of electricity, and unsightly aluminium plants are often built in regions of great natural Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry beauty. This can cause a conflict of interest between conservationists and industrialists. Recycling Increasing demand for aluminium, due to its amazing properties, means that even this very abundant resource cannot last forever. This couple with the ever growing problems of waste disposal, have led to considerable interest in recycling waste. Recycling has a number of possible advantages: uses only about 5% of the energy used in its extraction from bauxite. avoids some of the environmental problems in the extraction of aluminium from the bauxite. don’t have to find space to dump the unwanted aluminium if it wasn't recycled. Offsetting these (to a minor extent) are the energy and pollution costs in collecting and transporting the recycled aluminium. Questions a) Explain, with reference to economic considerations, why pure molten aluminium oxide is not used as the electrolyte. ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... b) Write an equation for the reaction at the cathode and indicate the physical state of the aluminium as it is formed. ............................................................................................................................... c) Write an equation for the reaction at the anode and explain why the regular replacement of anodes is necessary. ............................................................................................................................... Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry ............................................................................................................................... ............................................................................................................................... d) The unique properties of aluminium mean that every month new uses are being found for this amazing metal. Can you list some of the major uses of aluminium and given reasons why? Use Reason References A-level Chemistry pages 96-97 Chemistry in Context pages 274-276 Learning Objectives Candidates should be able to explain, including the electrode reactions, the industrial process of the extraction of aluminium from molten aluminium oxide/cryolite. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Section 3: Redox reactions Part 5: Purification of Copper Metallic copper obtained by smelting copper sulphide ores is 98-99% pure. For use in electrical wiring the impurities must be removed in order to increase the conductivity of the copper. This is done in an electrolysis cell. Electrolytic refining The purification uses an electrolyte of copper(II) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. The diagram shows a very simplified view of a cell. At the cathode, copper(II) ions are deposited as copper. At the anode, copper goes into solution as copper(II) ions. For every copper ion that is deposited at the cathode, in principle another one goes into solution at the anode. The concentration of the solution should stay the same. All that happens is that there is a transfer of copper from the anode to the cathode. The cathode gets bigger as more and more pure copper is deposited; the anode gradually disappears. In practice, it isn't quite as simple as that because of the impurities involved. Copper is found low in the reactivity series. This means it is not as easy to oxidise as more reactive metals like magnesium or zinc. Conversely, Cu2+ ions are much easier to reduce than Mg2+ or Zn2+. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry M(s) M2+(aq) + 2e- A process which is easy in one direction will be difficult in the reverse direction. Task 1 Explain why the purification process is not disturbed by the presence of a) metals higher than copper in the electrochemical series. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... b) metals lower than copper in the electrochemical series. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... Uses of copper Task 2 Can you match the properties of copper listed below with an appropriate use? Amongst other things copper is used for: ____________________: it is a very good conductor of electricity and is easily drawn out into wires. _______________: it doesn't react with water, and is easily bent into shape. _______________: it is a good conductor of heat and doesn't react with water. _______________: alloying produces a metal harder than either copper or zinc individually. Bronze is another copper alloy - this time with tin. _______________: many in the UK and Europe are made of copper-zincnickel alloys. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry References A-level Chemistry pages 96-98 Chemistry in Context pages 226, 273-276 Learning Objectives Candidates should be able to explain, including the electrode reactions, the industrial processes of the electrolytic purification of copper. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Section 4: Equilibria Part 1: Dynamic Equilibrium Reversible reactions A reversible reaction is one which can be made to go in either direction depending on the conditions. If you pass steam over hot iron the steam reacts with the iron to produce a black, magnetic oxide of iron called triiron tetroxide, Fe3O4. The hydrogen produced in the reaction is swept away by the stream of steam. Under different conditions, the products of this reaction will also react together. Hydrogen passed over hot triiron tetroxide reduces it to iron. Steam is also produced. This time the steam produced in the reaction is swept away by the stream of hydrogen. These reactions are reversible, but under the conditions normally used, they become one-way reactions. The products aren't left in contact with each other, so the reverse reaction can't happen. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Reversible reactions happening in a closed system A closed system is one in which no substances are either added to the system or lost from it. Energy can, however, be transferred in or out at will. Let’s imagine iron being heated in steam in a closed container. Heat is being added to the system, but none of the substances in the reaction can escape. The system is closed. As the triiron tetroxide and hydrogen start to be formed, they will also react again to give the original iron and steam. So, if you analysed the mixture after a while, what would you find? You would find that you had established what is known as a dynamic equilibrium. Dynamic equilibria Task 1 Try the task on the worksheet ‘Modelling dynamic equilibrium’. Results Round No. of blue No. of orange Round 1 7 2 8 3 9 4 10 5 11 6 12 No. of blue No. of orange You should have seen that the "reaction" is continuing all the time. The exact pattern of orange and blue is constantly changing. However, the overall numbers of orange and of blue squares remains remarkably constant - most commonly, 12 orange ones to 4 blue ones. Explaining the term "dynamic equilibrium" The reaction has reached equilibrium in the sense that there is no further change in the numbers of blue and orange squares. However, the reaction is still Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry continuing. For every orange square that turns blue, somewhere in the mixture it is replaced by a blue square turning orange. This is known as a dynamic equilibrium. The word dynamic shows that the reaction is still continuing. You can show dynamic equilibrium in an equation for a reaction by the use of special arrows. In the present case, you would write it as: blue orange It is important to realise that this doesn't just mean that the reaction is reversible. It means that you have a reversible reaction in a state of dynamic equilibrium. The "forward reaction" and the "back reaction" The change from left to right in the equation (in this case from blue to orange as it is written) is known as the forward reaction. The change from right to left is the back reaction. Position of equilibrium In the example we've used, the equilibrium mixture contained more orange squares than blue ones. Position of equilibrium is a way of expressing this. You can say things like: "The position of equilibrium lies towards the orange." "The position of equilibrium lies towards the right-hand side." If the conditions of the experiment change (by altering the relative chances of the forward and back reactions happening), the composition of the equilibrium mixture will also change. For example, if changing the conditions produced more blue in the equilibrium mixture, you would say "The position of equilibrium has moved to the left" or "The position of equilibrium has moved towards the blue". Thinking about reaction rates This is the equation for a general reaction which has reached dynamic equilibrium: Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Task 2 Think about how the concentrations of reactants and products will change with time. Use this to plot, using the same axes, the reaction rate against time. Task 3 Complete questions 2 and 1 of worksheet ‘Problems for 7.1’. A summary A dynamic equilibrium occurs when you have a reversible reaction in a closed system. Nothing can be added to the system or taken away from it apart from energy. At equilibrium, the quantities of everything present in the mixture remain constant, although the reactions are still continuing. This is because the rates of the forward and the back reactions are equal. If you change the conditions in a way which changes the relative rates of the forward and back reactions you will change the position of equilibrium - in other words, change the proportions of the various substances present in the equilibrium mixture. References A-level Chemistry pages 119-121 Chemistry in Context pages 289-294 Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Learning Objectives Candidates should be able to explain, in terms of rates of the forward and reverse reactions, what is meant by a reversible reaction and dynamic equilibrium. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Section 4: Equilibria Part 2: Le Chatelier’s Principle Le Chatelier’s Principle Le Chatelier's Principle is a useful guide to help you work out what happens when you change the conditions, i.e. concentration, pressure and temperature, of a reaction in dynamic equilibrium. Be aware: it doesn't explain anything. Put simply, Le Chatelier’s Principle states that: If a system is at equilibrium, and a change is made in any of the conditions, then the system responds to counteract the change as much as possible. Using Le Chatelier's Principle with a change of concentration Suppose you have an equilibrium established between four substances A, B, C and D. What would happen if you changed the conditions by increasing the concentration of A? According to Le Chatelier, the position of equilibrium will move in such a way as to counteract the change. That means that the position of equilibrium will move so that the concentration of A decreases again - by reacting it with B and turning it into C + D. The position of equilibrium moves to the right. This is a useful way of converting the maximum possible amount of B into C and D. You might use it if, for example, B was a relatively expensive material whereas A was cheap and plentiful. Task 1 What would happen if you changed the conditions by decreasing the concentration of C? This is essentially what happens if you remove one of the products of the reaction as soon as it is formed. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry ....................................................................................................................................................... ....................................................................................................................................................... Using Le Chatelier's Principle with a change of pressure This only applies to reactions involving gases: What would happen if you changed the conditions by increasing the pressure? According to Le Chatelier, the position of equilibrium will move in such a way as to counteract the change. That means that the position of equilibrium will move so that the pressure is reduced again. Pressure is caused by gas molecules hitting the sides of their container. The more molecules you have in the container, the higher the pressure will be. The system can reduce the pressure by reacting in such a way as to produce fewer molecules. In this case, there are 3 molecules on the left-hand side of the equation, but only 2 on the right. By forming more C and D, the system causes the pressure to reduce. Increasing the pressure on a gas reaction shifts the position of equilibrium towards the side with fewer molecules. Task 2 What happens if the number of molecules on both sides of the equilibrium reaction is the same? ....................................................................................................................................................... ....................................................................................................................................................... Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Using Le Chatelier's Principle with a change of temperature For this, you need to know whether heat is given out or absorbed during the reaction. Assume that our forward reaction is exothermic (heat is evolved): This shows that 250 kJ is evolved (hence the negative sign) when 1 mole of A reacts completely with 2 moles of B. For reversible reactions, the value is always given as if the reaction was one-way in the forward direction. The back reaction (the conversion of C and D into A and B) would be endothermic by exactly the same amount. What would happen if you changed the conditions by increasing the temperature? According to Le Chatelier, the position of equilibrium will move in such a way as to counteract the change. That means that the position of equilibrium will move so that the temperature is reduced again. Suppose the system is in equilibrium at 300°C, and you increase the temperature to 500°C. How can the reaction counteract the change you have made? How can it cool itself down again? To cool down, it needs to absorb the extra heat that you have just put in. In the case we are looking at, the back reaction absorbs heat. The position of equilibrium therefore moves to the left. The new equilibrium mixture contains more A and B, and less C and D. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Summary Increasing the temperature of a system in dynamic equilibrium favours the endothermic reaction. The system counteracts the change you have made by absorbing the extra heat. Decreasing the temperature of a system in dynamic equilibrium favours the exothermic reaction. The system counteracts the change you have made by producing more heat. Le Chatelier's Principle and catalysts Catalysts have sneaked onto this page under false pretences, because adding a catalyst makes absolutely no difference to the position of equilibrium, and Le Chatelier's Principle doesn't apply to them. This is because a catalyst speeds up the forward and back reaction to the same extent. Catalysts are still useful because they speed up the rate at which a reaction reaches dynamic equilibrium. Task 3 Complete questions 3 to 6 of worksheet ‘Equilibrium problems’. References A-level Chemistry pages 121-125 Chemistry in Context pages 305-309 Learning Objectives Candidates should be able to state Le Chatelier’s Principle and apply it to deduce qualitatively (from appropriate information) the effects of changes in temperature, concentration or pressure, on a system at equilibrium. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Section 4: Equilibria Part 3: Equilibrium constant Here we introduce a new concept, that of an equilibrium constant, and illustrate how this constant can be used: to provide a quantitative measure of the extent of a reaction; to determine the position of equilibrium. The equilibrium constant Kc at constant temperature An equilibrium expression may be written for any reaction. Consider the general equilibrium equation: aA + bB → cC + dD The expression for the equilibrium constant in terms of concentrations is: Kc [C ]c [ D]d [ A]a [ B]b where a, b, c and d are the number of moles of the species A, B, C and D which appear in the balanced equation for the equilibrium, and [ ] denotes a concentration in mol dm-3. Task 1 Write expressions for Kc for the following reactions: 1. 2. 3. 4. 5. 2NO(g) + C2H6(g) 2HI(g) CO2(g) + CH3COOH(l) + Cambridge A-level Centre O2(g) C2H4(g) + H2(g) H2O(l) C2H7OH(l) 2NO2(g) H2(g) + I2(g) HCO3 (aq) + CH3COOC3H7(l) H+(aq) AS Chemistry Unit 2: Physical Chemistry Units of Kc The units of Kc vary. It depends on the expression for Kc for the particular reaction you are studying. It is possible for Kc to have no units! Task 2 For each of the examples in Task 2 give the units of Kc. Calculating and using Kc The calculation of Kc is best illustrated by means of examples. Worked example 1. When 1.0 mol each of ethanol and ethanoic acid are mixed together at a fixed temperature, the reaction mixture at equilibrium is found to contain 0.66 mol of ethyl ethanoate. a. b. c. d. e. Write an equation for the reaction between ethanol and ethanoic acid. (1) The total volume of the reaction mixture is 0.10 dm3. Calculate the equilibrium concentrations of ethyl ethanoate and ethanol in the reaction mixture. (2) Write an expression for the equilibrium constant, Kc, for this reaction. (1) Calculate the value of the equilibrium constant for this reaction at this fixed temperature. (2) This reaction is affected by the addition of a few drops of conc. sulphuric acid. Suggest a possible role for the sulphuric acid added. State the effect of sulphuric acid on the rate of reaction and on the value of the equilibrium constant. (3) Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry The equilibrium constant Kp at constant temperature The composition of a solution is usually described in terms of the concentration of its components. Consequently the equilibrium constant is also expressed in concentration terms. It is difficult to describe the composition of a gas mixture in terms of concentrations; the volume of a solution is fixed whereas the volume of a gas is not. A more natural measure is the pressure. The quantity of each gas in an equilibrium mixture is described in terms of the pressure that it exerts – its partial pressure. The equilibrium constant can be expressed in terms of these partial pressures: it is given the symbol Kp. Partial pressure The partial pressure of a gas in a mixture of gases is the pressure it would exert if it alone occupied the total volume occupied by the gas mixture. Dalton’s law of partial pressures states that: The total pressure exerted by a mixture of gases is the sum of the partial pressure of the gases. Dalton’s law can be rephrased in terms of the mole fractions of each of the components of the mixture of gases. The mole fraction x of a component A in a gas mixture is defined as: The partial pressure is related to mole fraction and total pressure ptot by: pA = xA x ptot Partial pressure terms are expressed in SI units as Pa or kPa. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry The equilibrium constant Kp Consider the general equilibrium equation: aA(g) + bB(g) cC(g) + dD(g) The expression for the equilibrium constant in terms of partial pressures is: Kp = p(C ) c p( D) d p( A) a p( B) b As with Kc, the units of Kp depend on the stoichiometry of the equilibrium reaction. Worked example 2 2 mol of phosphorus (V) chloride vapour are heated to 500 K in a sealed vessel. The equilibrium mixture at a pressure of 665kPa contains 1.2 mol of chlorine. Calculate the value of the equilibrium constant Kp for the decomposition of phosphorus (V) chloride into phosphorus (III) chloride. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Qualitative effects of changes in pressure, temperature and concentration The position of equilibrium and the value of the equilibrium constant We have already seen that the effects of changes in reaction conditions can be described using Le Chatelier’s Principle. Once the effect on the position of equilibrium is known, it is relatively easy to deduce the effect that this will have on the position of the equilibrium constant. Change in temperature Task 3 Consider the following reactions: H2(g) + N2(g) + I2(g) O2(g) ∆H = -9.6kJmol-1 ∆H = +180 kJmol-1 2HI(g) 2NO(g) Use your scientific knowledge to complete the table below: ∆H for reaction Exothermic Exothermic Endothermic Endothermic Change in Temperature Increase Decrease Increase Decrease Shift of Equilibrium Yield of Product Equilibrium constant Change in concentration Consider the following reaction: CH3COOC2H5(l) + H2O(l) CH3COOH(l) + C2H5OH(l) Task 4 Write the equation for the equilibrium constant in the space below. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry If more water is added, Le Chatelier’s Principle tells us that the equilibrium is displaced to the right. In terms of the equilibrium constant, an increase in [H2O] is countered by a decrease in [CH3COOC2H5(l)] and a simultaneous increase in both products. In other words the original value of Kc is restored. N.B. If the reactants or products are gases, a change in the partial pressure of any gaseous species is equivalent to a change in the concentration of that species. Summary At a given temperature the value of the equilibrium constant Kc is fixed. If the concentration of any species involved in the equilibrium is changed, then the concentrations of the other species will change so that the value of Kc remains constant. Change in pressure Changes in total pressure have a significant effect on the composition of a mixture at equilibrium but only if the reactions involve gases. Increased pressure always shifts the equilibrium towards the side with fewer moles of gas. Although the position of equilibrium shifts, the value of Kp and that of Kc remains the same, whatever the pressure. You are not expected to know why at this stage. Task 5 a) The following reaction was allowed to reach equilibrium: 2D(aq) + E(aq) F(aq) The initial amounts of the reactants present in 1.00 dm3 of solution were 1.00 mol D and 0.75 mol E. At equilibrium, the amounts were 0.70 mol D and 0.60 mol E. Calculate the equilibrium constant, Kc. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry b) A mixture contained 1.00 mol of ethanoic acid and 5.00 mol of ethanol. After the system had come to equilibrium, a portion of the mixture was titrated against 0.200 mol dm-3 sodium hydroxide solution. The titration showed that the whole of the equilibrium mixture would require 289cm3 of the standard alkali for neutralisation. Find the value of Kc for the esterification reaction. Task 6 Complete questions on the worksheet ‘Equilibrium constants’. References A-level Chemistry pages 127-133 Chemistry in Context pages 305-308 Learning Objectives Candidates should be able to: deduce expressions for equilibrium constants in terms of concentrations, Kc, and partial pressures, Kp. deduce whether changes in concentration, pressure or temperature or the presence of a catalyst affect the value of the equilibrium constant for a reaction. calculate the values of equilibrium constants in terms of concentrations or partial pressures from appropriate data. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry calculate the quantities present at equilibrium, given appropriate data. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Section 4: Equilibria Part 4: Equilibria of importance We will now tie together all of our ideas about kinetics and equilibria by studying two industrially important reactions; the Haber process for the fixation of atmospheric nitrogen and the Contact process for the manufacture of sulphuric acid from sulphur. Task In groups of 3, use the information on pages 133-136 of A-level Chemistry and pages 310-315 of Chemistry in Context to complete the table below. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Equilibria of importance Process Main product Contact Main uses of product Balanced equation for main reaction/s 1. 2. 3. Catalyst Is the equilibrium reaction exothermic or endothermic? Optimum conditions for highest yield Cambridge A-level Centre Haber AS Chemistry Unit 2: Physical Chemistry Actual conditions used Why are these conditions chosen? Points of interest Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Summary of the Haber Process A flow scheme for the Haber Process looks like this: Ammonia is made by the Haber process from nitrogen and hydrogen: The forward reaction (the production of ammonia) is exothermic, and involves a decrease in the number of moles of gas. An application of Le Chatelier’s Principle shows that the forward reaction should be assisted by a low temperature and a high pressure. At low temperature, the rate of attainment of equilibrium is low. At high temperature, the position of equilibrium is over to the left. A compromise temperature is adopted, and a catalyst is employed to speed up the attainment of equilibrium concentrations. The conditions employed in the industrial plants are: 250-1500 atm, a temperature between 400 and 500oC, iron with a metal oxide promoter as catalyst. The ammonia is removed as it is formed so that the reaction mixture does not reach equilibrium. The yield is about 10% and unreacted gases are recycled. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry References A-level Chemistry pages 133-136 Chemistry in Context pages 310-315 Learning Objectives Candidates should be able to describe and explain the conditions used in the Haber process and the Contact process, as examples of the importance of an understanding of chemical equilibrium in the chemical industry. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Section 4: Equilibria Part 4: Acid and Base Equilibria We recognise that hydrochloric acid is an ‘acid’ because of the things it does. Task 1 Can you list the properties of an acid below? Chemists try to explain chemical properties in terms of what goes on at the level of atoms, molecules and ions. One of the most useful explanations of acid-base behaviour was put forward by Arrhenius in 1884. He said that: Acids are substances which produce hydrogen ions in solution. Bases are substances which produce hydroxide ions in solution. This theory allowed chemists to explain neutralisation as a reaction between these hydrogen and hydroxide ions to produce water. Limitations of the theory Hydrochloric acid is neutralised by both sodium hydroxide solution and ammonia solution. In both cases, you get a colourless solution which you can crystallise to get a white salt. These are clearly very similar reactions. Task 2 Can you write balanced equations for these reactions? Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry In the sodium hydroxide case, hydrogen ions from the acid are reacting with hydroxide ions from the sodium hydroxide - in line with the Arrhenius theory. However, in the ammonia case, there don't appear to be any hydroxide ions! You can get around this by saying that the ammonia reacts with the water it is dissolved in to produce ammonium ions and hydroxide ions: This is a reversible reaction, and in a typical dilute ammonia solution, about 99% of the ammonia remains as ammonia molecules. Nevertheless, there are hydroxide ions there, and we can squeeze this into the Arrhenius theory. However, this same reaction also happens between ammonia gas and hydrogen chloride gas. In this case, there aren't any hydrogen ions or hydroxide ions in solution - because there isn't any solution. The Arrhenius theory wouldn't count this as an acid-base reaction, despite the fact that it is producing the same product as when the two substances were in solution. That's silly! It is more useful to refer to acids as PROTON DONORS and bases as PROTON ACCEPTORS. This theory of H+ transfer is known as the Bronsted-Lowry theory. The reaction in which this occurs is called an acid-base reaction. Notice that this means an acid cannot act in isolation. In this reaction, the HCl transfers H+ to NH3. The HCl is behaving as an acid, and the NH3 is behaving as a base. (Notice that this is not a redox reaction – can you state why?) Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Task 3 In terms of subatomic particles, describe an H+ ion. ................................................................................................................................................ Solutions of acids and bases Hydrogen chloride is a gas and contains HCl molecules. Water is almost totally made up from H2O molecules. Yet hydrochloric acid, a solution of hydrogen chloride in water, readily conducts electricity, so it must contain ions. There must be a reaction between the hydrogen chloride molecules and the water molecules which produces these ions. Task 4 Can you write an equation for this reaction below? In this reaction, H2O is behaving as a base. You may not have come across the ion with the formula H3O+. It is called the oxonium or hydronium or hydroxonium ion. It is very common ion and is present in every solution of an acid in water. Task 5 Can you draw a diagram to explain how the H2O bonds to H+? Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry The H3O+ ion can itself act as an acid – it can donate H+ and turn into an H2O molecule. The familiar properties of acidic solutions are all properties of the H3O+ ion. You will often see the formula H3O+(aq) shortened to H+(aq) and the dissociation of HCl(aq) into ions represented by: HCl(aq) → H+(aq) + Cl-(aq) An alkali is a base that dissolves in water to produce hydroxide ions, OH-. Some alkalis such as sodium and potassium hydroxide, already contain hydroxide ions, whereas others, such as ammonia and sodium carbonate, form OH-(aq) ions when they react with water. Task 6 Can you write an equation to show the reaction of the carbonate ion with water? In this reaction, H2O is acting as an acid. Reaction of acids and bases in aqueous solution according to the Bronsted-Lowry scheme almost always involve the transfer of protons under equilibrium conditions. Consider the reaction between ammonia and hydrochloric acid in aqueous solution. This can be best be described by the following equilibrium: NH3(aq) + H3O+(aq) NH4+(aq) + H2O(aq0 Can you suggest why this is the best description of this reaction? Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry In the forward reaction, the base NH3 accepts a proton from the acid H3O+. In the reverse reaction, the base H2O accepts a proton from the acid NH4+. The acid NH4+ and the base NH3 are a conjugate acid-base pair because they are related by the transfer of a proton. Similarly, the acid H3O+ and the base H2O are a conjugate acid-base pair. In the reverse reaction, water is acting as a base. Here is a system you have seen before: The most fundamental proton transfer equilibrium in water involves one water molecule, acting as a Brønsted-Lowry acid, donating a proton to another water molecule, acting as a Brønsted-Lowry base H 2O + H 2O H 3O + OH- + This explains why pure water is a (poor) conductor of electricity. Task 7 a) What is the conjugate acid of: i) CH3COOii) HSO4- iii) NH3 iv) OH- ? b) What is the conjugate base of: i) HCl ii) H3O+ iii) HSO4- iv) NH4+ ? Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Amphoteric substances A substance which can act as either an acid or a base is described as being amphoteric. Strong and weak acids When an acid dissolves in water, a proton (hydrogen ion) is transferred to a water molecule to produce a hydroxonium ion and a negative ion depending on what acid you are starting from. In the general case . . . These reactions are all reversible, but in some cases, the acid is so good at giving away hydrogen ions that we can think of the reaction as being one-way. The acid is virtually 100% ionised. For example, when hydrogen chloride dissolves in water to make hydrochloric acid, so little of the reverse reaction happens that we can write: At any one time, virtually 100% of the hydrogen chloride will have reacted to produce hydroxonium ions and chloride ions. Hydrogen chloride is described as a strong acid. A strong acid is one which is virtually 100% ionised in solution. Other common strong acids include sulphuric acid and nitric acid. Explaining the term "weak acid" A weak acid is one which doesn't ionise fully when it is dissolved in water. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Ethanoic acid is a typical weak acid. It reacts with water to produce hydroxonium ions and ethanoate ions, but the back reaction is more successful than the forward one. The ions react very easily to reform the acid and the water. At any one time, only about 1% of the ethanoic acid molecules have converted into ions. The rest remain as simple ethanoic acid molecules. Most organic acids are weak. Hydrogen fluoride (dissolving in water to produce hydrofluoric acid) is a weak inorganic acid that you may come across elsewhere. pH is a measure of the number of H+ in solution. Task 8 Which would have the lower pH, a 1.0M solution of HCl or a 1.0M solution of CH3COOH? Can you explain why? ............................................................................................................................................................ ............................................................................................................................................................ .................................................................................................................................................... ............................................................................................................................................................ The reactions of acids with metals, metal oxides and metal carbonates Task 9 Can you write balanced equations for the reaction of HCl with i) calcium ii) strontium oxide, and iii) barium carbonate. Cambridge A-level Centre AS Chemistry Unit 2: Physical Chemistry Which species have you missed out and why?......................................................................... ............................................................................................................................................................ Task 10 Complete SAQ 7.13 on page 140 of your textbook. References A-level Chemistry pages 136-142 Chemistry in Context pages 198-200 Learning Objectives Candidates should be able to: • show understanding of, and use the Bronsted-Lowry theory of acids and bases. • explain qualitatively the differences in behaviour between strong and weak acids and bases and the pH values of their aqueous solutions in terms of the extent of dissociation. Cambridge A-level Centre