File - Chemistry - Summer 2015
advertisement

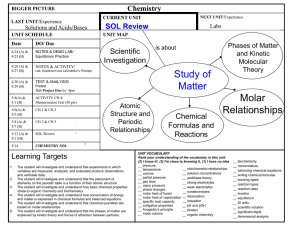
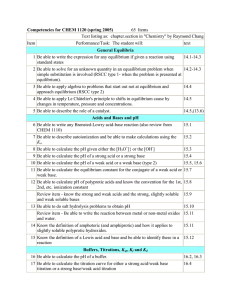
Pooyan Haghighat Chemistry – Place Cartier Summer 2015 (July 2nd to August 14th) Time: Mondays through Fridays 8:30am-12:00pm Classroom: 3, except for labs Teacher: Pooyan Haghighat Email: phaghighat@lbpearson.qc.ca Website: pooyanchemistry2015.weebly.com Contact: please contact through website Please come to me ASAP if you have any questions or concerns, if you need special accommodations, or if you haven’t finished math 416 or physical science. Please remember that respect and responsibility are the two main criteria for continued participation in this class. Also, absence without excuse and late arrivals will not be accepted. I look forward to working with you! Course Outline CHEMISTRY BOOK 1: CHEM5041 Properties of the gases Definitions of different gases in terms of its temperature, pressure, number of moles and mass Factors affecting the volume of a gas Kinetic theory of gases Real gas versus ideal gas Definition of pressure Avogadro’s hypothesis Charles’s law Gay Lussac’s law Boyle’s law General gas law Ideal gas law Dalton’s law (partial pressure law) Molar volume of a gas Volumetric mass of a gas CHEMISTRY BOOK 2: CHEM5042 Review of physical science 436 Endothermic and exothermic dissolutions (chemical reactions and distinctions) Molar heat and enthalpy change (ΔH) Enthalpy diagrams The factors that can influence the rate of reaction Maxwell-Boltzmann Distribution The three states of matter at two levels (macroscopic and microscopic) Phase changes graphs Heat capacity and molar heat (calculations) Quantity of heat Qsurroundings and Qsystem The calorimeter Hess’s law CHEMISTRY BOOK 3: CHEM5043 Le Châtelier’s principle: pressure, temperature and concentration Titration between an acid and a base Equilibrium constant value and its expression The ICE system Factors affecting a system in equilibrium Concentrations at equilibrium Systems in equilibrium and not in equilibrium Oxidation reactions Reduction reactions Redox reaction and its difference in potential Spontaneous and not spontaneous reactions Oxidation-Reducction couples Cathode and anode Electrochemical cell/Voltaic cell Pooyan Haghighat Course schedule Chem. 5041 – Gases Start date: Thursday, July 2nd Lab exam: Boyle’s law (20%) Monday, July 13th Theory exam (80%) Tuesday, July 14th Chem. 5042 – Chemical reactions 1 Start date: Wednesday, July 15th Lab exam: Heat of reaction (25%) Monday, July 27th Theory exam (80%) Tuesday, July 28th Chem. 5043 – Chemical reactions 2 Start date: Lab exam: Titration (30%) Theory exam (70%) Wednesday, July 29th Wednesday, August 12th Thursday, August 13th There are no retakes for the labs. If you fail a lab, you fail the book it is associated with.