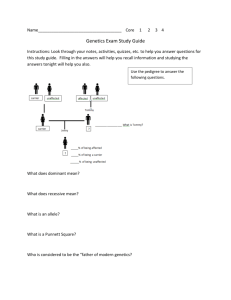
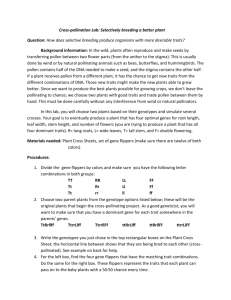
Abiy Thesis edited
advertisement

GENOTYPE x ENVIRONMENT INTERACTION AND STABILITY OF EARLY MATURING SORGHUM [Sorghum bicolor (L.) Moench] GENOTYPES IN ETHIOPIA M.Sc. THESIS ABIY LEGESSE KIBEBE OCTOBER 2015 HARAMAYA UNIVERSITY, HARAMAYA GENOTYPE X ENVIRONMENT INTERACTION AND STABILITY OF EARLY MATURING SORGHUM [Sorghum bicolor (L.) Moench] GENOTYPES IN ETHIOPIA A Thesis Submitted to the Postgraduate Program Directorate (School of Plant Sciences) HARAMAYA UNIVERSITY In Partial Fulfilment of the Requirements for the Degree of MASTER OF SCIENCE IN AGRICULTURE (PLANT BREEDING) By Abiy Legesse Kibebe October2015 Haramaya University HARAMAYA UNIVERSITY ii Postgraduate Program Directorate We hereby certify that we have read and evaluated this Thesis titled ̔ Genotype X Environment Interaction and Stability of Early Maturing Sorghum (Sorghum bicolor (L.) Moench) Genotypes in Ethiopia’ prepared under our guidance by Abiy Legesse. We recommend that it be submitted as fulfilling the thesis requirement. Firew Mekbib (PhD) Major Advisor Asfaw Adugna (PhD) Co-Advisor –––––––––––––––––– Signature –––––––––––––––––– Signature ––––––––––––––––– Date ––––––––––––––––– Date As members of the Board of Examining of the Final MSc Thesis Open Defense Examination, We certify that we have read and evaluated the thesis prepared by Abiy Legesse, and examined the candidate. We recommend that the thesis be accepted as fulfilling the Thesis requirement for the degree of Master of Science in Agriculture (Plant Breeding). ––––––––––––––––––––– Chairman ––––––––––––––––––––– Internal Examiner ––––––––––––––––––––– External Examiner –––––––––––––––––––– Signature –––––––––––––––––––– Signature –––––––––––––––––––– Signature –––––––––––––––––––– Date –––––––––––––––––––– Date –––––––––––––––––––– Date iii STATEMENT OF AUTHOR By my signature below, I declare and affirm that this thesis is my own work. I have followed all ethical and technical principles of scholarship in the preparation, data collection, data analysis and compilation of this thesis. Any scholarly matter that is included in the thesis has been given recognition through citation. This thesis is submitted in partial fulfillment of the requirements for the award of MSc degree in Plant Breeding at Haramaya University. The thesis is deposited in the Haramaya University’s Library and is made available to borrowers under the rules of the library. I solemnly declare that this thesis has not been submitted to any other institution anywhere for the award of any academic degree, diploma or certificate. Brief quotations from this thesis may be used without special permission provided that accurate and complete acknowledgement of the source is made. Requests for permission for extended quotations from, or reproduction of, this thesis in whole or in part may be granted by the Head of the School or Department when in his or her judgment the proposed use of the material is in the interest of scholarship. In all other instances, however, permission must be obtained from the author of the thesis. Name: Abiy LegesseSignature: –––––––––––––––––––– Date: October 2015 School: Plant Science, Haramaya University iv ACRONYMS AND ABBREVIATIONS AMMI Additive Main effect and Multiplicative Interaction ANOVA Analysis of Variance ASV Additive Main effect and Multiplicative Interaction Stability Value CSA Central Statistical Agency DF Degree of Freedom EMSG Early Maturing Sorghum Genotype GE Genotype by Environment GEI Genotype by Environment Interaction ICRISAT International Crop Research Institute for Sami-Arid Tropics IPCA Interaction Principal Component Analysis MET Multi-environment Trial MS Mean Squares PCA Principal Component Analysis RCBD Randomized Complete Block Design SAS Statistical Analysis System SS Sum of Squares v BIOGRAPHICAL SKETCH The author, Abiy Legesse Kibebe, was born in West Shoa Zone, Ambo town on May 1983. He attended his elementary education at Ambo Betekhnet Elementary School, and his junior and secondary school education at Ambo Senior Secondary School. He joined the Ambo College of Agriculture and graduated with diploma in General Agriculture in 2005. He began his first degree study at Ambo University directly after completion of his diploma and completed with BSc degree in Crop Production in March 2009. Following his BSc Degree graduation, he was employed in Agricultural Office of North Shoa Zone, as a seed science expert at Basonaworana Woreda for two months. Then, he joined Amhara Agricultural Research Institute at Debre Brihan Agricultural Research Center as a Junior Researcher I in June 2009, and worked there for four years and three months. In October 2006, he joined Haramaya University, Postgraduate Program Directorate to follow MSc Degree in Plant Breeding. vi ACKNOWLEDGMENTS I thank Amhara Agriculture Research Institute (ARARI) for providing the opportunity and funds for the study. I wish to express my deepest appreciation to my research advisors, Dr. Firew Mekbib and Dr. Asfaw Adugna for their genuine guidance, provision of references and constructive comments and encouragements to finalize the research work on time. I would like to extend my special thanks to Dr. Ribka G/Tsadek and Mr. Daniel Admasu for their valuable comments and edition of this manuscript. I am highly indebted to all the sorghum improvement program staff members of Errer Agricultural Research Sub-center, Sirinka Agricultural Research Center, Mieso Agricultural Research Sub-center, Debre Brihan Agricultural Research Center and Melkassa Agricultural Research Center for their assistance in field management, data collection and moral support. I also extend my thanks to all staff members of Debre Brihan Agricultural Research Center who directly or indirectly helped me during the implementation of the work, and especially to Mr. Getaw Cheregn. Last but not least, I would like to express my love and great thanks to my mother, father and sisters, my wife Teklil Alemayehu including all her amazing families and all my friends who gave courage and hospitality to me during the study period. vii TABLE OF CONTENT STATEMENT OF AUTHOR iii ACRONYMS AND ABBREVIATIONS iv BIOGRAPHICAL SKETCH v ACKNOWLEDGMENTS vi TABLE OF CONTENT vii LIST OF TABLES ixx LIST OF TABLES IN THE APPENDICES xx ABSTRACT xi 1. INTRODUCTION 1 2. LITERATURE REVIEW 4 2.1.Origin and Adaptability of Sorghum 4 2.2.Botany and Taxonomy of Sorghum 4 2.3.Constraints of Sorghum Growth and Development 5 2.4.Genotype x Environment Interaction 6 2.5.Concept of Stability 9 2.6.Genotype x Environment Interaction and Stability Analysis 10 2.6.1. Regression Coefficient and Deviation Mean Square 11 2.6.2. Additive Main Effect and Multiplicative Interaction (AMMI) 13 2.6.3. Additive Main Effect and Multiplicative Interaction Stability Value 16 3. MATERIALS AND METHODS 18 3.1.Description of the Study Area 18 3.2.Plant Materials 18 3.3.Experimental Design and Crop Management 19 3.4.Data Collection and Sampling Techniques 20 3.5.Data Analyses 21 3.5.1.Analysis of Variance for Each Location and Combined Over Locations 21 Continued 3.5.2.Stability Analysis 3.5.2.1.Eberhart and Russell’s joint regression model 22 22 3.5.2.2.The additive main effects and multiplicative interaction (AMMI) method 22 3.5.2.3.AMMI’s stability value (ASV) 4. RESULTS AND DISCUSSION 4.1.Analysis of Variance 23 24 24 4.1.1Single Location ANOVA 24 4.1.2Combined Analysis of Variance 25 4.2.Genotypes Mean Performance 28 4.3.Genotype x Environment Interaction Analysis of Variance 35 4.3.2. Genotype x Environment Interaction Analysis of Variance by AMMI Model 4.4.Stability Analysis 37 38 4.4.1. Stability Analysis by Eberhart and Russel's Model 38 4.4.2. Yield Stability Using ASV 40 5. SUMMARY AND CONCLUSIONS 43 6. REFERENCES 46 7. APPENDIX 58 ix LIST OF TABLES Table Page 1 18 Agro-ecological features of the experimental locations 2 19 Description of sorghum genotypes tested at four locations during 2014 main cropping season 3 Analysis of variance for days to emergence, flowering, and to maturity, plant 24 height, stand count at harvest, grain yield, grain filling period; and grain filling rate of twenty two early maturing sorghum genotypes tested at four locations during 2014 cropping season 4 Combined ANOVA for grain yield (ton/ha) and the percentage sum of squares 26 of the twenty two early maturing sorghum genotypes tested at four locations 5 Mean squares and coefficient of variations of yield, plant height; phenological 27 traits and grain filling rate of twenty two early maturing sorghum genotypes tested at four locations during the 2014 main cropping season 6 Means for days to emergence, grain yield (ton/ha) and grain filling rate of 29 Early Maturing Sorghum Genotypes tested at four locations 7 Mean plant height, stand count at harvest and grain filling period of twenty 33 two early maturing sorghum genotypes tested at Errer, Kobo, Mieaso and Shewa Robit during the 2014 main cropping season 8 Means for days to emergence, flowering and maturity, plant height, stand 34 count at harvest, grain yield, grain filling period and grain filling rate of twenty two early maturing genotypes at Errer, Kobo, Mieso and Shewa Robit 9 Analysis of Variance by Eberhart and Russel's Model of early maturing 36 sorghum genotypes on mean grain yield (ton/ha) tested at four locations. 10 AMMI analysis of variance for grain yield (ton/ha) of early maturing sorghum 37 genotypes tested at four locations during the 2014 main cropping season. 11 Eberhart and Russell’s (1966) stability parameters of early maturing sorghum 39 genotypes tested at four locations. 12 IPCA1 and IPCA 2 scores; and ASV for the twenty two early maturing sorghum genotypes sorted on mean yield (ton/ha) evaluated at four locations 47 x LIST OF TABLES IN THE APPENDICES Page Appendix 1 Mean value of yield (ton/ha), phenological traits and grain filling rate of 56 early maturing sorghum genotypes for the data collected at Errer during the 2014 main cropping season. 2 Mean value of grain yield (ton/ha), phenological traits and grain filling rate 57 of early maturing sorghum genotypes for the data collected at Kobo during the 2014 main cropping season. 3 Mean value of grain yield (ton/ha), phenological traits and grain filling rate 58 of early maturing sorghum genotypes for the data collected at Mieso during the 2014 main cropping season. 4 Mean value of grain yield (ton/ha), phenological traits and plant height of 59 early maturing sorghum genotypes for the data collected at Shewa Robit the during 2014 main cropping season. 5 Means for phenological traits and plant height and of early maturing 60 sorghum genotypes tested at four locations during the 2014 main cropping season. 6 The interaction principal component analysis 1 and 2 scores for the four 61 sites, sorted on environmental mean yield 7 Total monthly rainfall (mm) and mean monthly temperature (0C) of the four test locations during the main cropping season 61 xi Genotype x Environment Interaction and Stability of Early Maturing Sorghum [Sorghum bicolor (L.) Moench] Genotypes in Ethiopia ABSTRACT The yield performance of sorghum cultivars is highly influenced by environmental factors and genotype x environment interaction; therefor interaction is the major concern to plant breeders to develop improved cultivars. Twenty two early maturing sorghum genotypes were evaluated at four locations using randomized complete block design with the objectives of estimating the magnitude and nature of genotype x environment interaction for grain yield and other traits, and to determine the stability of genotypes for grain yield in lowland areas of Ethiopia. Phenological, plant height; grain yield, grain filling rate and stand count at harvest data were recorded. The combined analysis of variance revealed that the significant effect of locations on all the measured traits, while the interaction is significantly influences grain yield, grain filling rate and days to emergence. This showed that genotypes were inconsistent for grain yield across the testing locations. Joint linear regression analysis of variance revealed that the genotype x environment interaction was non-linear type and the pooled deviations were highly significant against pooled error. Genotype 2001 MS 7003, 2001 MS 7015, 2005 MI 5060 and 2005 MI 5066 were relatively stable and high yielders. The AMMI analysis of variance showed that the environment, genotype and interaction sum squares contributed 74.19 %, 6.86 % and 18.98 % to the treatment sum squares for grain yield respectively.In addition the first two IPCAs and interaction residual are significant. The first two IPCAs accounted for a total of 78.60 % of the interaction sum square. Due to the significant result of the interaction residual AMMI one and two biplot are not necessary. In both the genotype x environment interaction ANOVA,the results indicating the observed yield variation among genotypes were due to location and interaction rather than differences of genetic potential of genotypes. Results ofASV parameter showed the five most widely stable and high yielder genotypes are 2001 MS 7015, 2005 MI 5064, 2005 MI 5066, 2001 MS 7007 and 2001 MS 7003. Genotype 2001 MS 7003, 2001MS 7015 and 2005 MI 5060 are selected with both stability parameters as a high yielder and stable, and therefore, are the promising materials. Generally, this study showed the importance of testing early maturing sorghum genotypes for their yield and stability across diverse lowland areas of Ethiopia. Key words: AMMI, ASV, early maturing, Joint linear regression, lowland, sorghum 1. INTRODUCTION Sorghum [Sorghum bicolor (L.) Moench] belongs to the family Poaceae, and the genus Sorghum. It is principally a self-pollinating short day cereal, grown mainly for its grain. Depending on the genotype, panicle type, and wind direction and velocity the degree of out crossing reaches up to 30 % (Perlman and Sleeper, 1995). Domesticated sorghum is a diploid (2n = 2x = 20) C4 grass with a high photosynthetic efficiency and is a tropical origin. Sorghum originated in Africa, more precisely in Ethiopia, between 5000 and 7000 years ago (ICRISAT, 2005). Ethiopia is rich in different races of wild and cultivated sorghums. Nowadays, it is widely cultivated in different parts of Ethiopia. Firew (2009) states that Ethiopia is the primary center of origin and hence, center of diversity for sorghum. Sorghum is now widely found in the dry areas of Africa, Asia, Americas and Australia (Dickonet al., 2006). Sorghum is an important staple food crop for millions of people and animal feed across the world. Currently, large part of sorghum production areas in Ethiopia fall under the arid and semiarid regions that are characterized by high rainfall variability and low soil water storage capacity. In these areas, sorghum is grown as one of the major food cereals. Sorghum grain has high nutritive value, with 70-80 % carbohydrate, 11-13 % protein, 2-5 % fat, 1-3 % fiber, and 1-2 % ash (Prasad and Staggenborg, 2009). It has been utilized in various forms such as for making local bread (Injera) and for preparation of local alcoholic beverages (tela and areke). In addition, sorghum stalks and leaves are an increasingly important source of dry season feed for livestock, source of energy for cooking their daily foods, and as housing and fencing material In Ethiopia, grain crops constitute the majority of the annual total agricultural crop production at the country level. Total area coverage and production of grain crop in the country covers about 12.41 million hectares and 251, 536, 62.390 ton, respectively. Out of the total grain crop area, 79.38 % (9,848,745.96 hectares) was under cereals. From the total area of grain crops sorghum (13.52 %) is the third widely cultivated cereal after tef (24.31 %) and maize (16.08 %). Sorghum (15.22 %) is forth in its production after maize (25.81 %), tef (17.57 %), and wheat (15.60%). Oromia, Amhara and Tigray regions are the major three sorghum producers in the country. Out of the total sorghum area harvested in 2014 main cropping season, Oromia region accounts 39.92 % (669,575.97 hectares), Amhara and Tigray regions contributed 33.31 % (558,827.95 hectares) 2 and 12.82 % (215,111.82 hectares), respectively. The large share of national sorghum production was from Oromia that is about 43.72 % (16, 739, 20.87 ton), Amhara 29.64 % (11, 350, 11.41 ton) and Tigray 14.27 % (5, 463, 22.53 ton). From Oromia region Eastern and Western Hararge, and from Amhara region South Welo and North Shewa are among the major producers, that covers 114,028.52, 123,897.29, 43,260.60 and 137,037.07 hectares of land; and 257,652.55, 310,036.50, 109,145.47 and 313,470.13 ton of sorghum production, respectively. The national sorghum production is still low (2.28 ton/ha) and in major sorghum growing regions Oromia 2.5 ton/ha, Tigray 2.54 ton/ha and Amhara 2.03 ton/ha were obtained (CSA, 2014). In Ethiopia sorghum is predominantly cultivated in dry areas that cover nearly 66% of the total area of the country, sorghum production in this area is mainly dependent on seasonal rainfall; its production is being limited mainly by water stress due to low and variable rainfall between and with the seasons (Geremew et al.,2004). In these areas there is variability among the growing environments and genotypes are performing differently in different environments (Asfaw, 2007). Limitation of improved high yielding and stable varieties in these parts of the country are considered as one of the major constraints of sorghum production. In multi-environment trials the phenotype of an individual in each test environment is a measure of an environment main effect, a genotype main effect, and the genotype by environment interaction (GEI) (Yan and Tinker, 2005). GEI results from a change in the relative rank of genotype performance or a change in the magnitude of differences between genotype performances from one environment to another. GEI affects breeding progress because it complicates the demonstration of superiority of any genotype across environments and the selection of superior genotypes (Ebdon and Gauch, 2002).Genotype x environment interaction is the major concern to plant breeders for developing improved cultivars. Traits that are of economic relevance may be related to polygenic characteristics, and influenced by environment. Typically, environment explains 80 % or higher of the total yield variation; however, it is genotype and genotype x environment interaction that are relevant to cultivar evaluation (Yan et al., 2002). The GE interaction reduces the correlation between phenotype and genotype and selection progress. The yield performance of sorghum genotypes in different lowland environments of Ethiopia is not always the same; it is highly influenced by environmental factors. In sorghum breeding 3 programs it is difficult to select genotypes that produce high yields and stable in multi-location trials (Asfaw, 2007). The occurrence of environmental causes of variation over the genetic effects does not suggest that the importance of genotype should be minimized ( Faisal and Aisha, 2011). So that a considerable attention should be given to the effect of GEI in the plant breeding programs (Ghazy et al. 2012). In the presence of GEI, one of the options open to the breeder is to use stability analyses to identify the most high yielding and stable sorghum genotype. The stability of a cultivar refers to its consistency in performance across environments and affected by the presence of GEI. For a genotype to be released as a variety for cultivation, stability of performance is one of the most desirable properties. Yield stability is a complex product of genetic yield potential and that would mean minimum variation among environments for a particular genotype (Chahal and Gosal, 2002). There are remarkable inconsistencies with the univariate stability estimates, which create difficulty in recommendation of cultivars for production (Asfaw, 2007). However, the multivariate approach, the additive main effect and multiplicative interaction (AMMI) model is better for partitioning the GEI into the causes of variation (Asfaw, 2007). Genotype x environment interaction for yield has been studied in several crops. Several research institutions are actively working and able to screen out sorghum varieties for yield that resist harsh environmental conditions and produce consistently better grain yield. Hence, the national and regional sorghum improvement programs have been released a number of stable early maturing sorghum genotypes for the moisture deficit lowland areas of Ethiopia. However, information on the effect of GEI for the yield performance of early maturing sorghum genotypes under different Ethiopian low land growing conditions is limited. Hence, the objectives of the present study were to: 1. Assess the magnitude and nature of GEI for grain yield and related traits, and 2. Determine the stability of early maturing sorghum genotypes for grain yield at lowland areas of Ethiopia. 4 2. LITERATURE REVIEW 2.1. Origin and Adaptability of Sorghum Sorghum is one of the crops for which Ethiopia has been credited as being a Vavilovian center of origin (Vavilov, 1951) and/or diversity (Dillon et al., 2007). Ethiopia is one of the Vavilovian centers of origin/diversity for sorghum (Vavilov, 1951). Sorghum originated in Africa, more precisely in Ethiopia, between 5000 and 7000 years ago (ICRISAT, 2005). From there, it was distributed along the trade and shipping routes around the African continent, and through the Middle East to India at least 3000 years ago. It then journeyed along the Silk Route into China (Dicko et al., 2006). It was first taken to North America in the 1700-1800's through the slave trade from West Africa and was re-introduced in Africa in the late 19th century for commercial cultivation and spread to South America and Australia. Sorghum is now widely found in the dry areas of Africa, Asia (India and China), the Americas and Australia (Dicko et al., 2006). It is an economically, socially and culturally important crop grown over a wide range of ecological habitats in Ethiopia, in the range of 400-3000 m.a.s.l (Teshome et al., 2007). Sorghum is the single most important cereal in the lowland areas because of its drought tolerance (Kebede, 1991). 2.2. Botany and Taxonomy of Sorghum The genus Sorghum has been classified into five subgenera: Eu-sorghum,Chaetosorghum, Heterosorghum, Para-sorghum and Stiposorghum. Although this classification is convenient, however it does not stand for evolutionary relationships (Dillon et al., 2004). The Eu-sorghum comprises the cultivated species S. Bicolor (L.) Moench and its subspecies are drummondii, arundinaceum, and wild species includes S. xalum Parodi, S. halepense (L.) Pers. and S. propinquum (deWet, 1978). The Eu-sorghum section is originated from Africa or Asia Doggett (1976), DuVall and Doebley (1990). Sections Chaetosorghum and Heterosorghum consist of S. macrospermum and S. Laxiflorum and both of these species are annuals and polyploids (Lazarides et al., 1991). Section Stiposorghum includes ten species (Lazarides et al. 1991). Parasorghum Section is comprised seven African, Asian, Australian and Central American species. The basic number of chromosome of species in each section is five. The species belong to Para sorghum and Stiposorghum are mostly diploid (2n = 20), however a few species are tetraploid or hexaploid. 5 Sorghum includes three species, S. halepense, S. propinquum and S. bicolor. Sorghumhalepense is also known as Johnson grass, derived from a natural cross between S. arudinaceum and S. propinquum (Doggett, 1976). Sorghum propinquum is a perennial species related to S. bicolor (Sun et al., 1994). By using Harlan and deWet‘s system which is based on spikelet morphology, Sorghum bicolor has been classified into five races. The five basic races of Sorghum bicolor are bicolor, guinea, caudatum, kafir and durra; and ten intermediate races under S. bicolor. It is a cereal of a remarkable genetic variability; with more than 30,000 selections present in the world genetic collections (Assefa and Staggenborg, 2010). Most of the tropical sorghums are short day plants and their response to day length is an important adaptation (Prasad and Staggenborg, 2009). Grain sorghum belongs to the family of Poaceae, tribe Andopogoneae, sub-tribe Sorghinae, and genus Sorghum. In 1794, Moench established the genus Sorghum and brought the sorghums under the name S. bicolor. All cultivated sorghum belongs to Sorghum bicolor subsp. bicolor (Dicko et al., 2006). 2.3. Constraints of Sorghum Growth and Development The productivity of grain sorghum is influenced by several abiotic and biotic constraints. Among the abiotic yield constraints (water, temperature and nutritional stresses), water will likely the primary yield constraint throughout the semi-arid tropics in the coming years (Ryan and Spencer, 2001). In addition, Assefa et al., 2010 reported, plant-available water, soil water content at planting, growing–season rainfall amount and distribution, crop management practices, and other climatic conditions highly affect the production of grain sorghum. Major biotic constraints to sorghum production include shoot fly, stem borer, head bug and aphid insect pests; grain mold anthracnosis, leaf blight and charcoal rot diseases; weed competition and the parasitic weed. Jones and Johnson (1991) reported that effect of stress due to environmental factors on final yield may depend upon the growth stage in which it occurs. Water stress has diverse effects on physiology and development of sorghum that determines its final yield depending on the development stage at which stress occurs. Assefa et al. (2010) found a 36 % sorghum yield reduction when water stress occurred during the vegetative stage. Prasad et al. (2008) reported lowered yield due to heat and drought stress 6 occurring during flowering and anthesis. More than 55 % yield reduction with water stress occurring during the reproductive stage Assefa et al. (2010). At this stage the yield reduction was caused by the failure of fertilization because of the impairment of pollen and ovule function. Moisture stress early in the season will limit panicle size and delay maturity. If the stress occurs later in the season, the seed size is greatly reduced Prasad et al. (2008). In addition, temperature stress can delay flowering, reduce stem and root growth, plant height, pollen and ovule viability, pollen number, stigma receptivity, seed number, seed filling duration, thus yield (Prasad and Staggenborg, 2009). 2.4. Genotype x Environment Interaction Genotype x environment interactions (GEI) is of major concern to plant breeders for developing improved cultivars. It refers to the differential responses of different genotypes across a range of environments. The phenotype of an individual is determined by the effects of its genotypes (G), the environment (E) surrounding it, and the interaction between the genotype of the individual and the environment (Yan and Tinker, 2005). Genotype x environment interactions is commonly observed by crop producers and breeders as the differential ranking of cultivar yields among locations or years. Quantitative traits, those which are controlled by several genes, are highly influenced by environmental factors. Most agronomically and economically important traits, such as grain yield, are quantitative or multigenic in nature. Experiments for such type of traits in single environments do not allow the drawing of general conclusions regarding the tested genotypes; therefore yield trials should typically be done on a number of varieties in a number of environments (reference). With regard to the comparison of plant materials in a set of multi-environment trial, the term genotypes refers to a cultivar with materials genetically homogeneous, such as pure lines or clones, or heterogeneous, such as open-pollinated population rather than to an individual’s genetic make-up. The term environment relates to the set of climatic, soil, biotic and management conditions in an individual trial carried out at a given location in one year or over several years. Purely the environmental effect, reflecting the different ecological potential of locations and management conditions, are not of direct concern for the breeding or recommendation of plant varieties. Genetic main effects provide the only information when GEI effects are absent. However, differences between genotypes may vary widely among 7 environments in the presence of GEI effects. In general, GEIs are considered as a hindrance to crop improvement in a target region (Kang, 1998). Comstock and Moll (1963) classified environment into macro-environment and microenvironment. Micro-environment is environment of a single plant, which is made up of all the things, other than genotype of a plant, which influence its development. Environments, that is potential or experienced within a given area and period of time, are collectively known as macroenvironment. A macro-environment is a population of micro-environments. The environment that organisms experience in one area as compared to the other in one period of time than in another is not the same. In order to distinguish among environmental source of variation that contributes to the GEI, Allard and Bradshaw (1964) created two terms as predictable and unpredictable environmental variation. Predictable variation includes all the permanent characters of environment such as general features of climate and soil type as well as those characters of environment that fluctuate in a systematic manner, such as the day-length. It also includes those aspects of environment which are determined by man and can, therefore, be fixed more or less at will, like planting date, and sowing density. The unpredictable variation includes fluctuations in weather, such as amount and distribution of rain fall and temperature. The phenotype of an individual is determined by both the genotype and the environment; these two effects are not always additive which indicates that GEI, are present. The GEIs result in inconsistent performances between the genotypes across environments. Significant GEI results from the changes in the magnitude of differences between genotypes in different environments or changes in the relative ranking of the genotypes Falconer (1952), Fernandez (1991). According to Baker (1990) and Cornelius et al. (1996) GEIs have been grouped in to crossover and non-crossover interactions. The differential response of cultivars to diverse environments is referred to as a crossover interaction when cultivar ranks change from one environment to another. A main feature of crossover interaction is intersecting lines in a graphical representation. If the lines do not intersect, there is no crossover interaction (Kang, 1998). Non-crossover (quantitative) interactions represent changes in magnitude of genotype performance, but rank order of genotypes across environments remains unchanged, i.e., genotypes that are superior in one 8 environment maintain their superiority in other environments. Non-crossover interactions may mean that genotypes are genetically heterogeneous but test environments are more or less homogeneous or that genotypes are genetically homogeneous but environments are heterogeneous. In crop breeding, the crossover interaction is more important than non-crossover interaction (Baker, 1990). Since, the presence of a crossover interaction has strong implications for breeding for specific adaptation, it is important to assess the frequency of crossover interactions (Singh et al., 1999). Different agricultural researches have been conducted in multi-location experiments, and in most of these experiments the mean square for GEI source of variation showed significant differences and the rank order of genotypes tested are different from one environment to the other. Genotype x environment interaction is one of the main complications in the selection of broad adaptation in most breeding programs. The phenotype of an organism is determined by the combined effect of the environment and the genotype which interact with one another. Numerous studies have shown that booth environmental and genetic factors are the cause for the interaction, but in some studies the large difference of genotypes or environments has been the real cause of the interaction Hagos and Fetien (2011), Mahnaz et al. (2013), Sewagegne et al. (2013). Domitruk et al. (2001) indicated that the analysis of variance procedure is a useful tool for estimating the existence and magnitude of GEI. In the multi environment trial, the combined analysis of variance is useful for estimating variance components related to different sources of variation, including genotypes, environment and GEI. In MET, environment explains 80 % or higher of the total yield variation (Yan andHunt, 2002). The environment factors that are contributing to the differences in mean grain yield across environments and years may include soil types, sowing dates, sunshine hours and amount of rainfall during the crop cycle (Dagnachew et al., 2014). Different authors have conducted their experiment on different crops and as they have reported in a multi environment trial for yield, the total variation of the contribution of environmental sum square takes the largest share Asfaw (2007) and Vangge et al. (2014) on sorghum genotypes; Muez et al. (2014) on malt barely; Akcuraet al. (2006) on durum wheat;Shrestha et al. (2012) on maize; Dagnachew et al. (2014) on triticale. 9 The effects of genotype and environment on sorghum grain were investigated using 15 sorghum genotypes grown during three years (2003–2005) at three different locations (Melkasa, Kobo, and Mieso) to investigate the effect of GEI on sorghum yield performance in the drought stressed parts of Ethiopia (Asfaw, 2007). The study results revealed that the environments and genotypes were diverse. The performance of genotypes in the various environments was different. The contribution of genotypes, environments and GEI were 5.9 %, 73.8 % and 20.3 % of the total sum of squares, respectively. The large sum of squares for environments indicated that the environments were diverse, with large differences among environmental means causing most of the variation in grain yield. The magnitude of the GEI sum of squares was 3.41 times larger than that of the genotypes, indicating, that there were substantial differences in genotype response across environments. Similarly, different authors have also conducted a multi environment trial for yield in the world to improve the yield potential and stability of crops. In most of the trials, the results revealed that, the yield potential of genotypes have been significantly affected by their genetic capability, environmental variation of the growing area and the interaction of the two Vangge et al. (2014); Muez et al. (2014); Akcura et al. (2006); Yonas (2014). However, some of the others have got significant effects the growing environments and GEI, but genetically genotypes variation was insignificant Jipan (2013); Dagnachew et al.(2014). 2.5. Concept of Stability The term stability is sometimes used to characterize a genotype, which shows a relatively constant yield, independent of change in environmental conditions. On the basis of this idea, genotypes with a minimal variance for yield across different environments are considered to be stable (Sabaghnia et al., 2006). The basic cause of differences between genotypes in their yield stability is the wide occurrence of GEI, which means that the ranking of the genotype depends on the particular environmental conditions where it is grown. Different concepts and definitions of stability have been described over the years (Lin et al., 1986; Becker and Leon, 1988). Two basic phenotypic stability concepts are distinguished as the biological or static concept, and the agronomic or dynamic concept. The biological concept of stability refers to the constant performance of a genotype over a wide range of environments. According to Becker and Leon (1988), in biological stability,a genotype possesses unchanged 10 performance regardless of variation of the environments, thus, implying that its variance among environments is zero. On the other hand, dynamic stability, also termed as agronomical concept of stability, implies that a stable genotype should always give high yield expected at the level of productivity of the respective environments, which means thata variety with GEI as small as possible (Becker, 1981). Becker and Leon (1988) stated that all stability procedures based on quantifying GEI effects belong to the dynamic stability concept. This includes the procedures for partitioning the GEI of Wricke’s (1962) ecovalence and Shukla’s (1972) stability of variance, procedures using the regression approach such as proposed by Finlay and Wilkinson (1963), Eberhart and Russell (1966) and Perkins and Jinks (1968), as well as non-parametric stability statistics. Lin et al. (1986) identified three concepts of stability: Based on type one stability concept a stable genotype possesses an unchanged performance regardless of any variation of the environmental conditions. Parameters used for this type of stability are coefficient of variability used by Francis and Kannenburg (1978) for each genotype as a stability parameter and the genotypic variances across environments. Becker and Leon (1988) called this stability a biological concept of stability. Type two stability concepts select a stable genotype, if a genotype has no deviations from the general response to environments and thus permits a predictable response to environments. A regression coefficient by Finlay and Wilkinson (1963) and Shukla (1972) stability variance can be used to measure this type of stability. Becker and Leon (1988) called this stability agronomic concept of stability. Type three stability concept refers to a genotype that has a small mean deviation. Therefore a genotype is considered to be stable if the residual mean square from the regression model on the environmental index is small. Breeding for broad adaptability requires a different interpretation and approach to the stability analysis procedure than breeding for specific adaptability. According to Becker and Leon (1988) this is part of the agronomic stability concept. Methods to describe this type of stability are the methods of Eberhart and Russell (1966) and Perkins and Jinks (1968). 2.6. Genotype x Environment Interaction and Stability Analysis Selection for superior genotypes based on yield per se at a single location in a year may not be very effective (Shrestha et al., 2012). Thus, evaluation of genotypes for stability of performance under varying environmental conditions for yield has become an essential part of any breeding 11 program. Several methods have been proposed to analyze GEI and phenotypic stability including: Finlay and Wilkinson (1963) regression coefficient; Eberhart and Russell (1966) regression coefficient and deviation from regression; Pinthus (1973) coefficient of determination; Wricke (1962, 1964) ecovalence; Shukla (1972) stability variance parameter; Multivariate analysis methods (Principal component analysis, Principal coordinate analysis , Factor analysis, cluster analysis and Additive main effects and multiplicative interaction (AMMI). 2.6.1. Regression Coefficient and Deviation Mean Square Joint linear regression is a model used for analyzing and interpreting the non-additive structure (interaction) of two-way classification data. In the model proposed by Eberhart & Russell (1966), sum of the mean square attributable to environments and GEI are partitioned into environments (linear), GE (linear) and deviation from regression (pooled deviation over all the genotypes). This model uses the marginal means of the environments as independent variables in the regression analysis and restricts the interaction to additive form. The method divides the (g1) (e-1) degree of freedom for interaction into g-1 degree of freedom for heterogeneity among genotype regressions and the remainder (g-1) (e-2) for deviation. Eberhart and Russell’s defined a stable genotype as one with average response to the environment. They further said that a large GEI limits progress from selection, and to reduce this, the environments have to be stratified to make them more similar. In their study, they found that GEI was large, and they decided to select stable genotypes that interact less with the environments in which they were grown, and used only the more stable genotypes for the final stages of testing. Eberhart & Russell’s model portioned the interaction sum of squares into predictable (linear) and unpredictable components to characterize adaptation of the different genotypes across different environments. A regression coefficient approximating one coupled with deviation from regression of zero indicates average stability (Eberhart and Russell, 1966). Regression coefficient was considered as an indication of the response of the genotype to varying environment. If the regression coefficient is not significantly different from unity, the genotype is adapted to all environments. Regression coefficient values above one describe genotypes with higher sensitivity to environmental change (below average stability) and greater specificity of adaptability to high yielding environments. A regression coefficient below one (regression 12 coefficient values is negative) provides a measurement of greater resistant to environmental change (above average stability), and thus increases the specificity of adaptability to poor environments. In Eberhart and Russell’s model, when the mean square for pooled deviation is significant but mean square for GE (linear) is non-significant, variation in the performance of genotypes is entirely unpredictable. On the other hand, significance of mean squares for pooled deviations, when mean square for GE (linear) is also significant, implies that part of the variability is unpredictable in nature. Generally, significance of pooled deviation from zero will invalidate the linear prediction. But, if the pooled deviation is non-significant, the performance of a genotype for a given environment may be predicted. Accordingly, a variety whose performance can be predicted (𝑖. 𝑒. , Sd2𝑖 = 0) is said to be stable. In another study, Kenga et al. (2003) conducted the multi environment trial on sorghum hybrids and parental lines, and obtained significant mean square due to environment (linear), significant G x E (linear) interaction, and also significant pooled deviations from regressions. Therefore, the fluctuation in performance of genotypes grown in various environments is not fully predictable. In addition to this, they observed that the large portion of the sum of squares of GEI effects was accounted for by the deviations from regression than linear regression. Therefore, he noted that the magnitudes of GEI effects in this set of materials are largely due to differential non-linear responses of genotypes to varying environment; thus Sd2𝑖 parameters become important. Contrary to the above, Akcura et al. (2006) tested the mean squares of linear and non- linear against pooled error mean squares for grain yield of durum wheat. The result of the test prompted him to say that the linear component was highly significant, indicating that the predictable components shared GEI. Preponderance of linear GEI is of great practical importance, implying that there are differences among linear regression coefficients for each genotype. According to Eberhart and Russell (1966), mean squares of linear have to be tested against the pooled deviation if and only if the pooled deviation against the pooled error is significant. Otherwise, the mean squares of the linear part of the interaction are tested against the pooled error as they did. 13 Becker and Leon (1988) the result of the analysis could be non-linear type of interaction, because of insignificant GE (linear), reflecting lack of genetic differences among genotypes for their response to varying environments. While pooled deviations were highly significant against pooled error they show that the differences in stability were due to deviation from linear regression only Khan et al.(1988) on sorghum; Ashraf et al. (2001) on wheat. In these situations, the above method detect the most suitable and stable varieties over different environments based on bi value of genotypes which is almost near to unity, non-significant deviation from regression and above average grain yield of genotypes. 2.6.2. Additive Main Effect and Multiplicative Interaction (AMMI) Analysis of variance (ANOVA) is merely an additive model in which the GEI is a source of variation, but its key effects are not analyzed. In contrast, principal component analysis (PCA) is a multiplicative model and, therefore, does not present additive main effects for the environment nor genotype. However, the newly developed AMMI analysis includes ANOVA and PCA in a unified approach that can be used to analyze multiple yield trials Kang and Gauch (1996); Oliveira et al. (2014); Zobel et al. (1988). AMMI can treat both the additive main effect and multiplicative interaction component employing the ANOVA and IPCA, respectively (Gauch and Zobel, 1996). AMMI uses ANOVA to test the main effects of genotypes and environments, and PCA to analyze the residual multiplicative interaction between genotypes and environments to determine the sum of squares of the GEI, with a minimum number of degrees of freedom. Because ANOVA and PCA are parts of the AMMI model, this model is likely more suitable for characterizing the GEI (Zobel et al., 1988). Furthermore, AMMI biplot analysis is considered as an effective tool to diagnose GEI patterns graphically Gauch and Zobel (1996); Thillainathan and Fernandez (2001); Yuksel et al. (2002). AMMI quantifies the contribution of each genotype and environment to the sum square of GEI, and provides an easy graphical interpretation of the results, by the biplot technique that simultaneously classifies genotypes and environments Kempton (1984); Zobel et al. (1988). Therefore, with this technique, one can readily identify productive cultivars with wide adaptability or mega environments, as well as delimit the agronomic zoning of cultivars with specific adaptability and identify environments in which to conduct tests Kempton (1984). 14 The biplot display of PCA scores plotted against each other provides visual inspection and interpretation of GEI components. Mixing biplot display and genotypic stability statistics enable genotypes to be grouped based on similarity of performance across diverse environments (Thillainathan and Femandez, 2001). It also clearly separates main and interaction effects that present agricultural researchers with different kinds of opportunities, and the model provides agronomically meaningful interpretation of the data (Ebdon and Gauch, 2002). The results of AMMI analysis are useful in supporting breeding program decisions such as specific and broad adaptation, and selection of environment (Gauch and Zobel, 1997). There are several AMMI models characterized by number of significant PC axis ranging from zero (AMMI-0, i.e. additive model) to a minimum between (g – 1) and (l – 1). The full model, with the highest number of PC axes, provides a perfect fit between expected and observed data. Models including one (AMMI-1) or two (AMMI-2) PC axes are usually the most appropriate where there is significant GEI. Due to their simplicity, they provide a notable reduction of dimensionality for the adaptation patterns relative to observed data. Kampton (1984) pointed out that on the biplot results from the AMMI analysis, the following points should be considered: the center of biplot shows the mean of a genotype or an environment, a long distance of a genotype or an environment from the center of biplot indicates a large interaction with that genotype or environment, the long length of a genotype on the environmental vector reveals more deviation from the mean and vice versa and the angle between the vectors of a genotype and an environments shows that the interaction is positive or negative. The AMMI1 biplot, showing main effects means on the abscissa and IPCA 1 values as the ordinates, genotypes and/or environments that appear almost on a perpendicular line have similar means and those that fall almost on a horizontal line have similar interaction patterns. Genotypes and/or environments with large IPCA 1 scores (either positive or negative) have high interactions, whereas genotypes and/or environments) with IPCA 1 scores near zero have small interactions (Crossa et al., 1990). The effect of GE on sorghum yield performance in the drought stressed parts of Ethiopia was investigated using 14 sorghum hybrids and one released open-pollinated sorghum variety grown at eight different environments; the environments being Melkasa and Mieso during 2003, 2004 15 and 2005 and Kobo during 2003 and 2004 (Asfaw, 2007). Because the GEI effect was significant for grain yield, using five univariate stability models, he tried to analyze the yield data of sorghum and compared for their effectiveness in partitioning the GEI into parameters that permit a study of phenotypic stability of the sorghum genotypes. According to this work, the three types of stability parameters declared different genotypes to be the most stable. This inconsistency in ranking of genotypes was stated as a problem to reach a conclusion on producing genotype recommendation. Because of this and the absence of considering the yield response of genotypes across environments while using clustering genotypes (Flores et al., 1998), Asfaw (2007) tried to solve all the problems using AMMI models. From the AMMI analysis of variance for grain yield, observed, the environments were diverse withthis large difference among environmental means causing most of the variation in grain yield of sorghum. In the AMMI analysis, he tried to use the first three IPCA. However, as a reason of the large portion of the interaction was explained by the first two IPCAs, GEI pattern is collected in the first principal components of analysis (Sneller et al., 1997; Zobel et al., 1988), the first two IPCA axes best explain the GE sum square and the remaining can be considered as noise, he used the first two IPCA. Graphically, from the AMMI 1 biplot graph unfavorable and favorable environments were identified based on their main effects. In his study, he identified the least interactive genotypes and environments, and lastly depending on the yield potential of genotypes as well as stability, he recommended four genotypes for the drought stressed sorghum growing areas (Asfaw, 2007). Sixteen lowland rice genotypes were evaluated at three locations of eight environments in north western Ethiopia from 2006 to 2008 to identify stable and high yielding genotypes for possible release (Sewagegn et al., 2013). To achieve the objective of the study, he exploited the AMMI model. AMMI analysis of variance indicated that the interaction was partitioned among the first four IPCA, which cumulatively captured 91.13 % of the total GEI. AMMI 1 biplot and AMMI 2 biplot were used as the tools to classify genotypes and environments and recommend the most stable genotypes. From AMMI 1 biplot, high yielder genotypes and favorable environments were selected using the mean yield of genotypes over location and mean yield of environments, respectively. The most interactive genotypes and environments were also identified from the AMMI 1 graph using the IPCA sore of the respective genotypes and environments. Using the IPCA 1 and IPCA 2 scores of both the additive factors graphically in the biplot two they selected genotypes for both favorable and unfavorable environments. Lastly, they concluded that almost 16 all of the evaluated genotypes were affected by the GEI effects, so that no genotype had superior performance in all environments. Therefore, they selected genotypes for specific adaptation (Sewagegn et al., 2013). Currently, plant breeders widely use AMMI stability model for the purpose of classifying environments to be either favorable or unfavorable group for that specific crop to allocate genotypes to either widely or specifically adaptation and to direct the countries breeding strategy. Accordingly, Asfaw et al. (2011) and Molla et al. (2013) on finger millet ; Alemida et al. (2014), and Human et al. (2011), on sorghum; Dagnachew et al. (2014) and Sunday et al. (2013) on triticale were conducted multi environment yield trial and analyzed their yield data using AMMI stability model. 2.6.3. Additive Main Effect and Multiplicative Interaction Stability Value Purchase (1997) developed the AMMI stability value (ASV) based on the AMMI model’s IPCA1 and IPCA2 (interaction principal components axes 1 and 2, respectively) scores for each genotype. ASV is the distance from the coordinate point to the origin in a two dimensional plot of IPCA1 scores against IPCA2 scores in the AMMI model. The ASV as described by Purchase (1997) is comparable with the methods of Shukla(1972), Wricke, (1962) and Eberhart and Russell (1966) in South African wheat (Purchase et al., 2000). In effect, the ASV is the distance from zero in a two dimensional scatter-gram of IPCA 1 scores against IPCA 2 scores. Since the IPCA 1 score contributes more to GEIsum square, to compensate for the relative contribution of IPCA 1 and IPCA 2 to the total GEI sum square, it has to be weighted by the proportional difference between IPCA 1 and IPCA 2 scores. The distance from zero is then determined by using the theorem of Pythagoras. The larger the ASV value, either negative or positive, the more specifically adapted a genotype is to certain environments. Smaller ASV values indicate more stable genotypes across environments (Purchase, 1997). To identify stable high yielder triticale genotypes, Dagnachew et al. (2014) used Eberhart and Russell’s model, AMMI, ASV mode and genotype selection index in one MET yield data of triticale. The stability analysis result of this work supported Purchase’s (1997) ASV model; the stable genotypes obtained from the above four stability models were almost similar. Based on their yield and stability results, two triticale genotypes were selected as a candidate for wide 17 adaptation release. These genotypes were among five genotypes that had lower ASV values (Dagnachew et al., 2014). In Ethiopia, yield stability of malt barley genotypes was measured using ASV of genotypes and other stability parameters. According to the ASV ranking of this trial, two malt barley genotypes were identified as the most stable with their lowest ASV values and one genotype was found as the most unstable with its high ASV value (Meuz et al., 2014). Vange et al. (2014), conducted an field experiment on improved sorghum genotypes during the 2009 and 2010 cropping seasons in four locations within the Southern Guinea Savanna. The result of the combined ANOVA for grain yield revealed significant differences with reference to genotype and highly significant differences with respect to the Environment and Environment X Genotype interactions. This indicating that there were tangible differences among the environments as well as the genotypes. As the interaction is significant showing that the relative performances of the genotypes were significantly affected by varying environmental conditions. Based on Eberhart and Russell (1966) stability parameters three genotypes were selected as stable and adapted to the test environments. From the result of this yield data genotypes were also identified for poor environments Vange et al. (2014). Almeida et al. (2014) evaluated grain yield of twenty five sorghum hybrid data collected from experiments conducted across seven locations of Brazil during 2011for stability and adaptability. To determine stability and adaptability Eberhart and Russell (1966) and AMMI (Zobel et al., 1988) statistical parameters are implemented. Eberhart and Russell model identified three sorghum hybrids as stable showed regression coefficients that were statistically higher than one and were a great fit to the model. In addition, one cultivar shows adaptability to unfavorable environments, although the fit to the model was low. The association between the AMMI and the Eberhart and Russell (1966) methods was very useful in explaining the performance of hybrids with adaptability to favorable environments. According to the AMMI model, the first two principal components were used. In consequence, due to the fact that the first two IPCAs explained 65.98% of the variance due to the GEI and the interaction residual is non-significant the biplot is constructed (Almeida et al., 2014). 18 3. MATERIALS AND METHODS 3.1. Description of the Study Area The field experiment was conducted during the 2014 main cropping season at four locations representing the dry lowland areas of Ethiopia where sorghum is widely grown. The research was conducted at Shewa Robit, Kobo, Mieso and Errer; which are found in North Shewa, North Wello, Western Harerghe and Eastern Harerghe, respectively. The detailed agro-ecological features of the locations are presented on Table 1. Table 1 Agro-ecological features of the experimental locations Locations Altitude (m.a.s.l) Average Rain fall Soil Type (mm) Geographic coordinates Average Temperature (ºC) Latitude Longitude Max. Min. Errer 1305 NA NA 45o 05’ 20’’ N 09 o14’33’’E NA NA Kobo 1450 673.4 Vertisol 12o 8’ 21’’ N 39o 18’ 21’’ E 34 13 Mieso 1470 856.8 Vertisol 16o 06N 37o 8E 35 8.3 Shewa Robit 1500 890.7 Vertisol 10o 35’ N 39o 93’E 36.23 12.05 Source:From annual reports ofMelkasa, Sirinka and Debre Brihan Agricultural Research Centers, NA = Not available 3.2. Plant Materials The experimental plant materials comprised of one released early maturing sorghum variety Melkam (released from Melkasa Agricultural Research Center for low moisture stress areas of the Ethiopian lowlands in 2009) as a standard check and twenty one advanced early maturing sorghum genotypes screened in preliminary yield trials. The detailed information about the materials is presented on Table 2. 19 Table 2 Description of sorghum genotypes tested at four locations during 2014 main cropping season Entry Code Genotypes Pedigree Seed Source 1 2001 MS 7003 Local Bulk(white)/SRN-39 MARC 2 2001 MS 7013 PGRC/E#222880/ICSV-1/KAT369-1 MARC 3 2001 MS 7015 PGRC/E#222880/ICSV-1/KAT369-1 MARC 4 2001 MS 7037 PGRC/E#222878/ICSV708 MARC 5 IESV 92084-DL IESV92084-DL ICRISAT 6 IESV 92168-DL IESV92168-DL ICRISAT 7 IESV 92199-DL IESV92199-DL ICRISAT 8 IESV 92057-DL IESV92057-DL ICRISAT 9 IESV 9027-DL IESV9027-DL ICRISAT 10 2001 MS 7007 CR:35:5/DJ1195/N13 MARC 11 2005 MI 5060 WSV387/P9403 MARC 12 2005 MI 5064 WSV387/P9404 MARC 13 2005 MI 5065 WSV387/P9405 MARC 14 2005 MI 5066 M36121/P9401 MARC 15 2005 MI 5069 M36121/P9402 MARC 16 2005 MI 5070 M36121/P9403 MARC 17 2005 MI 5075 3443-2-OP/P9401 MARC 18 2005 MI 5079 3443-2-OP/P9401 MARC 19 2005 MI 5081 3443-2-OP/P9403 MARC 20 2005 MI 5082 3443-2-OP/P9403 MARC 21 ICSR 24005 ICSR24005 ICRISAT 22 Melkam (Check) WSV387 MARC MARC= Melkasa Agricultural Research Center, ICRISAT= International Crop Research Institute for Sami-Arid Tropics. 3.3. Experimental Design and Crop Management The trial was laid out in randomized complete block design (RCBD) with three replications. The experimental plots consisted of 5 rows, each 5 m in length with 75 cm row to row and 15 cm 20 plant-to-plant spacing. The total area of each plot and the three harvestable middle-rows had a size of 18.75 m2 and 11.25 m2,respectively. Sowing was done by hand drilling. The seed rate for each plot was calculated as per the recommendation for row planting (10 kg/ha). Seeds were sown by hand drilling. Then, thinning was done two weeks after emergence to adjust plant to plant space. Nitrogen and phosphorus fertilizer applications were practiced in the form of urea (46 % N) and DAP (18 % N and 46 % P2O5) as the national sorghum improvement program have done. During planting, 100 kg/ha of DAP was applied in the seed furrow. Urea was applied as top dressing at the rate of 50 kg/ha at knee height stage. The field was kept free of weeds during the period of the experiment. All of the other recommended agronomic management practices such as land preparation and insect pest control were applied as required. 3.4. Data Collection and Sampling Techniques Data were collected from the central three rows and five randomly sampled plants based on the descriptors for sorghum (IBPGR/ICRISAT, 1993). Phenological data (emergence date, flowering date, and maturity date), morphological data (plant height), and data on yield (g/plot) and stand count at harvest were collected. The details of the data collection were as follow: Days to 50 % seedling emergence:the number of days from the date of sowing to the date at which 50 % of the seedlings in a plot were emerged. Days to 50 % flowering: the number of days from 50 % seedling emergence to the date at which 50 % of the plants in a plot started flowering. Days to maturity: the number of days from 50 % seedling emergence to the date at which 75 % of the plants in a plot are physiologically matured. Grain filling period:The numbers of days from days to 50 % flowering to days to 75 % physiological maturity were counted, and it includes watery ripe stage, milk stage, soft dough stage, hard dough stage and ripening stage.. Plant height (cm): Plant height was measured from five randomly sampled main plants from the three central rows at 75 % physiological maturity. The mean height from the five plants was then recorded for the plot. 21 Stand count at harvest:the total number of main plants in net plot area when 75 % of the total population in a plot was physiologically mature. Grain yield (kg/ha): after harvesting, the panicles from the three central rows of each plot were threshed cleaned and weighed. The plot yield (g/plot) was converted to kg/ha and ton/hectare. Grain filling rate (kg/ha/day): it is the ratio of grain yield (kg/ha) to grain filling period and calculated as follows: Grain yield (𝑘𝑔⁄ℎ𝑎) Grain Filling Rate= Grain Filling Period (days) 3.5. Data Analyses SAS, Spar 2.0 and Genestat statistical softwares were used to analyze the data. SAS 9.1 was performed to analyze all the collected data from individual locations and the combined data over locations. 3.5.1. Analysis of Variance for Each Location and Combined Over Locations Using the raw data collected to eight characters of 22 genotypes, which were grown at four locations, general analysis of variance (ANOVA) of RCBD was computed as outlined by Gomez and Gomez (1984). Before pooling the data over locations, Bartlett’s test of homogeneity of variance was adopted for the eight parameters to determine the validity of the combined analysis of variance of the data. This analysis revealed the homogeneity of error variance. Therefore, combined analysis of variance was done to determine the effects of the genotypes, locations and their first order interactions using mixed linear model. Genotypes were assumed to be fixed and environment effects random. Duncan’s multiple range test (DMRT) was used to determine the significance of differences among the genotype means for each character. The effects of genotypes, locations as well as their first order interaction were determined from the ANOVA using the following model: Yijk= µ + 𝐺𝑖 + 𝐸𝑗 + 𝐺𝐸𝑖𝑗 + 𝐵𝑗𝑘 + 𝑒𝑖𝑗𝑘 22 Where: µ is the grand mean, 𝐺𝑖 is the effect of the ith genotype, 𝐸𝑗 is the effect of the jth location, 𝐺𝐸𝑖𝑗 is the interaction of the ith genotype with the jth location, 𝐵𝑗𝑘 is the effect of the kth replication in the jth location, and 𝑒𝑖𝑗𝑘 is the random error. 3.5.2. Stability Analysis The following two analyses of the stability models were performed for grain yield (ton/ha) using Spar 2.0 and Genestat softwares. 3.5.2.1. Eberhart and Russell’s joint regression model Eberhart and Russell (1966) procedure involves the use of joint linear regression where the yield of each genotype is regressed on the environmental mean yield. Then, the behavior of the genotype was assessed by the model: 𝑌𝑖𝑗 = 𝜇𝑖 + 𝛽𝑖 𝐼𝑗 + δ𝑖𝑗 using Spar 2.0 statistical software. Where: Yij = the mean performance of the ith genotype in the jth environment, µi = the grand mean of the ith genotype over all the environments, βi = the regression coefficient which measures the response of the ith genotype on environmental index, Ij = the environmental index obtained by the difference between the mean of each environment and the grand mean and 𝛿𝑖𝑗 = the deviation from regression of ithvariety in the jth environment The pooled deviations mean square was tested against the pooled error mean square by the F-test to evaluate the significance of the differences among the deviations of genotypes being evaluated from their expected performances. As a result, in order to test the validity of the hypothesis that whether there is significant difference among the 22 genotypes with respect to their mean grain yields or not and whether there is significant difference among the regression coefficient or not, genotypes mean square and regression mean square were tested against the pooled deviation using the F-test. 3.5.2.2. The additive main effects and multiplicative interaction (AMMI) method Additive main effects and multiplicative interaction(AMMI) model was performed for the mean data of grain yield (ton/ha) from each location using Genestat statistical software. The AMMI model equation is given as: 23 N Y𝑖𝑗 = µ + α𝑖 + ß𝑗 + ∑ λ𝑛 γ𝑖𝑛 δ𝑗𝑛 + θij + εij n=0 Where:Yij = the mean yield of genotype i in environment j,µ = the grand mean,αi = the deviation of the genotype mean from the grand mean, βj = the deviation of the environment mean from the grand mean, λ n = the singular value for the IPCA n, N = the number of PCA axis retained in the model, γin = the PCA score of a genotype for PCA axis n,δjn = the environmental PCA score for PCA axis n, θij = the AMMI residual and Eij = the residuals. The degrees of freedom (DF) for the IPCA axis were calculated based on the following method (Zobel et al., 1988). DF = G + E – 1 – 2n; Where: G = the number of genotypes, E = the number of environments and n = the nth axis of IPCA. 3.5.2.3. AMMI’s stability value (ASV) In order to quantify and rank genotypes according to their yield stability, the additive main effect and multiplicative interaction effect stability value (ASV) was proposed by Purchase (1997). It was calculated using Microsoft excel (2007) by employing the following formula: √[𝐼𝑃𝐶𝐴1 𝑠𝑢𝑚𝑜𝑓𝑠𝑞𝑢𝑎𝑟𝑒𝑠(𝐼𝑃𝐶𝐴1 𝑠𝑐𝑜𝑟𝑒)]2 𝐴𝑆𝑉 = + (𝐼𝑃𝐶𝐴2 𝑠𝑐𝑜𝑟𝑒)2 𝐼𝑃𝐶𝐴2 𝑠𝑢𝑚𝑜𝑓𝑠𝑞𝑢𝑎𝑟𝑒 Where: ASV = AMMI’s stability value, IPCA1= interaction principal component analysis one, and IPCA I= interaction principal component analysis II. 24 4. RESULTS AND DISCUSSION 4.1. Analysis of Variance 4.1.1 Single Location ANOVA The separate ANOVA of the eight characters (grain yield, days to emergence, flowering and maturity, plant height, stand count at harvest, grain filling period and grain filling rate) for twenty two early maturing sorghum genotypes tested at Errer, Kobo, Mieso and Shewa Robit was presented on Table 3. The results of ANOVA for grain yield at each location showed the presence of genetic variation among the genotypes. The difference among the genotypes for grain yield are highly significant (P≤ 0.01) at Mieso, and very highly significant (P≤ 0.001) at Errer, Kobo and Shewa Robit (Table 3). This indicates that, at each location there are genetic variability among genotypes for grain yield. Similar results of significant effect of genetic base of genotypes on one of a multi environment yield trial growing environment for grain yield were reported by the previous works of Abubakar and Bubuche (2013), Ahmed et al. (2012), Fahri (2012), Mesfin et al. (2014), Tekle and Zemach (2014), Shrestha (2013). Table 3 Analysis of variance for days to emergence, flowering, and to maturity, plant height, stand count at harvest, grain yield, grain filling period; and grain filling rate of twenty two early maturing sorghum genotypes tested at four locations during 2014 cropping season. Loc. Errer Kobo Mieso Shewa Robit S.V. Df Gen. 21 DE 0ns Rep. Error Gen. Rep. Error Gen. Rep. Error Gen. Rep. Error 2 42 21 2 42 21 2 42 21 2 42 0ns 0 0ns 0ns 0 0.3ns 0.2ns 0.21 2.7** 1.1ns 0.6 DF 2.5ns DM 0.21ns Traits PH SCH 553.2** 237.6ns 1.9ns 4.04 28.7* 52.6* 12.4 13.1ns 11.8ns 11.5 33.1ns 56.8ns 26.1 0.02ns 0.21 13.1* 13.9ns 5.78 32.5ns 32.2ns 36.8 6.5ns 1.0ns 4.7 386.9ns 201.69 361.4* 23.5ns 194.5 409.3ns 177.7ns 293.1 254.2ns 865.5* 182.8 1160.3* 161.5 108.4ns 68.0ns 73.9 69.4ns 81.5ns 50.0 288.8* 206.8ns 80.3 GY 1.15*** GFP 1.9ns GFR 385.9*** 1.35* 0.16 0.91*** 0.10ns 0.13 0.52** 0.84* 0.16 1.60*** 0.79* 0.22 1.8ns 3.7 8.7ns 13.7ns 5.2 7.8ns 5.9ns 3.1 21.0ns 70.4ns 21.9 373.1* 53.35 656.7*** 203.16ns 108.07 192.17** 254.99* 64.78 414.45** 382.94ns 124.89 25 DE = Days to emergence (days), DF= Days to flowering (days), DM = Days to maturity (days), PH = Plant height (Cm), SCH = Stand count at harvest (number), GY = Grain yield (ton/ha), GFP = Grain filling period (days), GFR = Grain filling rate (%), *** = vary highly significant (P≤ 0.0001), ** = highly significant (P≤ 0.001), * = significant (P≤ 0.0=01) and ns = insignificant (P>0.05). The ANOVA result for seven traits (days to emergence, days to flowering, days to maturity, plant height, stand count at harvest, grain filling period and grain filling rate) of twenty two early maturing sorghum genotypes tested at Errer, Kobo, Mieso and Shewa Robit showed that the performances of genotypes for days to emergence, days to flowering, days to maturity, plant height and stand count at harvest were not uniform in all the locations. The observed numbers of days that genotypes spent to flower and mature were statistically different at Kobo. At Kobo, genotypic differences were significant (P≤ 0.05) for days to flowering, days to maturity, and plant height. Genotypes were also found to vary significantly (P≤ 0.01) for plant height at Errer. At Shewa Robit the mean square of genotypes for days to emergence and total number of sorghum stands at maturity revealed a highly significant (P≤ 0.01) and significant (P≤ 0.05) variation among genotypes, respectively (Table 3). From the seven measured traits genotypic differences were significant only for grain filling rate in all the experimental areas. The differences of grain filling rate among genotypes are very highly significant (P≤ 0.001) at Errer and Kobo, and highly significant (P≤ 0.01) at Mieso and Shewa Robit. In all the four locations, genotypes took similar period to fill their grain (Table 3). 4.1.2 Combined Analysis of Variance The combined ANOVA of the twenty two early maturing sorghum genotypes tested at four locations during 2014 main cropping season is presented on Table 4. The result revealed that there were significant (P≤ 0.001) differences among locations, but differences among the genotypes were not significant. This indicates the diversity of the growing conditions in the four locations and the lack of variability in the genotypes for grain yield performance. Significant effect of location on yield of sorghum varieties was reported by Asfaw (2007), Almeida et al. (2014), Maposa et al. (2010).The GEI was also very highly significant (p≤ 0.001), showing the difference in the response of genotypes at different environments. This result is in agreement with the findings of Almeida et al. (2014), Asfaw (2007), Kenga et al. (2003) differential 26 genotypic behavior in the environments. In the other study Dagnachew et al. (2014) obtained insignificant genotypic effect and very highly significant environmental and GEI effect on the yield of triticale. A significant GEI may be either a non-cross-over or cross-over type (Baker, 1990; Cornelius et al., 1996). In the present study, the interaction was of cross-over type as the ranking of genotypes for grain yield changed at every location (Appendix 1-4). Table 4 Combined ANOVA for grain yield (ton/ha) and the percentage sum of squares of the twenty two early maturing sorghum genotypes tested at four locations during 2014 main cropping season Source of Variation DF SS % SS MS Genotype 21 23.28 6.24 1.11ns Location 3 252.09 67.34 84.03*** Replications in Environments 8 6.17 1.67 0.77*** Genotype x Location 63 64.48 17.22 1.02*** Error 168 28.37 7.58 0.17 Total 263 374.39 DF = Digress of freedom, SS = Sum of squares and MS = Means of squares, *** = vary highly significant (P<0.0001), ** = highly significant (P<0.001), * = significant (P<0.0=01) and ns = non-significant (P>0.05). The result of the combined ANOVA showed that the total variation in yield was attributed to environmental (67.34 %), genotypic (6.24 %) and GEI (17.22 %) effects (Table 6). This indicates that the largest proportion of the variation was among the environments. Similar results of large environmental effects were also reported for sorghum genotypes by Asfaw (2007, 2008), Hagos and Fetien (2011), Mahnaz et al. (2013), Sewagegne et al. (2013). Therefore, high percentage of the environment component of variation is an indication that environment is the major factor that influence yield performance of sorghum genotypes in the dry lowlands of Ethiopia. The sum of squares of GEI was 2.76 times higher than that of the genotypes. The highest magnitude of the interaction as compared to the genotype component indicates that the grain yield performance of sorghum genotypes across environments was different (Asfaw 2007). 27 The effect of interaction on the grain yield of sorghum genotypes was large, indicating the needs for studying the nature of differential response of genotypes to environments up on selecting genotypes for grain yield. Significant GEI indicates that the effects of genotypes and environments are statistically nonadditive or the differences between genotypes depend on the environment. Hence, superior genotypes across environments cannot be selected based on their mean yield performance alone. As a result, there is a need to dissect the significant interaction effect into the components that are responsible for the variation. Therefore, to test the consistency of genotypes for grain yield performance across locations, the multi-location grain yield data should be subjected to different stability analysis methods. Combined ANOVA was made for days to emergence, flowering and maturity, plant height, stand count at harvest, grain filling period and grain filling rate (Table 5). Statistically significant differences (P≤ 0.05) among genotypes were found only for stand count (P≤ 0.01), plant height (P≤ 0.001) and grain filling period. This indicates that the presence of the effect of genetic differences for the above three traits. Differences among locations were highly significant for all traits, indicating the wide variation among locations had high effect for these traits of early maturing sorghum genotypes. Table 5 Mean squares and coefficient of variations of yield, plant height; phenological traits and grain filling rate of twenty two early maturing sorghum genotypes tested at four locations during 2014 main cropping season. Mean Squares Pooled Errer Traits (Df = 168) Genotype Location GLI Replication (Df=21) (Df=3) (Df= 63) (Df= 2) ns DE 0.7736291 306.3977273*** 0.7495791*** 0.3219697ns 0.20689 ns ns DF 26.9761905 1150.676768*** 16.816979 30.746212* 13.512085 ns ns ns DM 9.3881674 3809.11616*** 14.30928 11.76894 11.88799 ns ns PH 903.75281*** 853.10186** 224.7872 360.89822 218.04163 SCH 324.05267** 16013.56566*** 126.70058ns 379.14394ns 91.42172 ns GY 1.11186046 84.0271545*** 1.0229794*** 0.7698375*** 0.1686208 GFP 14.7373737* 5136.13636*** 8.19192ns 22.93182* 10.13023 ns GFR 399.098695 25327.92576*** 416.61119*** 303.55752** 87.773 DE = Days to emergence (days), DF = Days to flowering (days), DM = Days to maturity (days), PH = Plant height (Cm), SCH = Stand count at harvest (number), GY = Grain yield (tone/ha), 28 GFP = Grain filling period (days), GFR = Grain filling rate (%), *** = vary highly significant (P<0.0001), ** = highly significant (P<0.001), * = significant (P<0.0=01) and ns = non- significant (P>0.05). The GEI was very highly significant for days to emergence and grain filling rate only. The result of this indicates that genotypes took different days to emerge at different locations and also the grain filling rate potential of genotypes varied from location to location. Zahra et al. (2013) found that rate of 50 % germination was affected by temperature, genotype and their interaction. 4.2. Genotypes Mean Performance The performance of twenty two early maturing genotypes for days to emergence, grain yield and grain filling rate are highly affected by the combined effect of both genotype and growing conditions of locations. Therefore, the mean performance of genotypes for these three traits are compared based on the average number of days that genotype took to emerge, mean yield and average grain filling rate of genotypes obtained across the four tested locations. The mean emergence day, grain yield and grain filling rate of genotypes across location are presented in table 6. According to Vanderlip (1979) emergence is the first stage (Stage 0) of grain sorghum development; it is when the plant first breaks through the soil surface. Zahra et al. (2013) observed that differences existed among sorghum genotypes in germination. The average numbers of days that genotypes took to emerge are statistically similar. The average number of days across location is 7.41. Above half of the tested genotypes were emerged before the mean days of genotypes across the four locations. From the tested genotypes at four locations genotype ICSR 24005 (8.17 days) had maximum number of days to emerge (Table 6). In contrary genotype 2005 MI 5064 (7.08 days) had numerically minimum number of days to emerge. The difference of the two marginal days of genotype emergence is small, this result agrees with the ANOVA result that showed the statistical similarity of genotypes emergence date. Generally for germination of sorghum genotypes it may occur 5 to 10 days after planting (table 6). The time required for emergence depends on soil texture and temperature, moisture conditions, depth of planting, vigor of the seed and genotypes Zahra et al. (2013). 29 Table 6 Means for days to emergence, grain yield (ton/ha) and grain filling rate of Early Maturing Sorghum Genotypes tested at four locations during 2014 main cropping season. Genotypes Code Genotype DE GY (ton/ha) GFR 1 2001 MS 7003 7.17 3.34 71.13 2 2001 MS 7013 7.58 3.33 72.02 3 2001 MS 7015 7.42 3.30 68.38 4 2001 MS 7037 7.33 2.57 55.81 5 IESV 92084-DL 7.42 3.71 77.59 6 IESV 92168-DL 7.17 3.33 67.22 7 IESV 92199-DL 7.17 2.88 59.15 8 IESV 92057-DL 7.42 3.12 60.70 9 IESV 9027-DL 7.67 2.86 58.90 10 2001 MS 7007 7.67 3.47 67.62 11 2005 MI 5060 7.33 3.37 69.29 12 2005 MI 5064 7.08 3.68 74.32 13 2005 MI 5065 7.25 3.67 72.21 14 2005 MI 5066 7.25 3.35 69.42 15 2005 MI 5069 7.67 3.09 66.83 16 2005 MI 5070 7.33 3.23 65.16 17 2005 MI 5075 7.25 3.09 62.49 18 2005 MI 5079 7.25 3.00 62.87 19 2005 MI 5081 7.75 2.67 56.59 20 2005 MI 5082 7.50 3.25 64.52 21 ICSR 24005 8.17 3.31 65.70 22 Melkam (Standard check 7.25 3.49 70.38 Mean 7.41 3.23 66.29 CV (%) 6.14 12.70 14.13 DE = days to emergence, GY = grain yield, GFR = grain filling rate The average number of days that genotypes take to emerge at Errer, Kobo, Mieso and Shewa Robit was 8, 6, 8.47 and 10.20 days, respectively (Table 8). The average number genotypes 30 emergence day at Shewa Robit was statistically larger than the three locations. This might be due to the amount and occurrence of rain fain fall and temperature at the time of plantation. The major environmental factors that affect germination of sorghum genotypes are temperature (including soil temperature), moisture and soil texture. This author was also reported that, change in temperature regime from 25/22 to 11/8 °C caused reduction in germination percentage, soil with low temperature in few weeks after sowing reduces seed germination, emergence rate and seedling establishment Zahra et al. (2013). From the tested twenty two early maturing sorghum genotypes thirteen genotypes had higher grain yield than the grand mean (3.23 ton/ha): 2001 MS 7003 (3.34 ton/ha), 2001 MS 7013 (3.33 ton/ha), 2001 MS 7015 (3.30 ton/ha), IESV 92084-DL (3.71 ton/ha), IESV 92168-DL (3.33 ton/ha), 2001 MS 7007 (3.47 ton/ha), 2005 MI 5060 (3.37 ton/ha), 2005 MI 5064 (3.68 ton/ha), 2005 MI 5065 (3.67 ton/ha), 2005 MI 5066 (3.35 ton/ha), 2005 MI 5082 (3.25 ton/ha), ICSR 24005 (3.31ton/ha) and Melkam (3.49 ton/ha). The standard check was also among the high yielding genotypes. Maximum and minimum grain yield was obtained from genotype IESV 92084-DL (3.71 ton/ha) and 2001 MS 7037 (2.57 ton/ha), respectively (Table 6). Melkam (WSV 387) was officially released as early maturing sorghum variety in 2009 from Melkasa Agricultural Research Center for the dry lowland areas of Ethiopia (altitude having less than 1600). The result of this study compares the relative yield potential of Melkam with the rest 21 early maturing sorghum genotypes; the grain yield performance of the standard check was among the top high yielder genotypes at Kobo, Mieso and Shewa Robit; but at Errer it had significantly low yield than all the tested genotypes, except, the five lowest yielder genotypes (Table 6). Sorghum growing conditions of the four locations were quite different. The result of this study showed that the average yield of locations was 3.23 ton/ha. Except Shewa Robit (4.87 ton/ha) none of the three locations had mean yield above the grand mean (3.23 ton/ha). The highest mean of genotypes at this location might be due to variation in distribution of rain fall until the end of the growing period. The mean yield of genotypes at Errer (3.02 ton/ha), Kobo (2.72 ton/ha) and Mieso (2.32 ton/ha) were also statistically different (Table 8). The large variation of locations for grain yield might be due to the difference in total amount of rain fall at the growing season and at different growing stage of sorghum genotypes, temperature, and soil conditions. 31 The performance of genotypes for grain filling rate differed from location to location. Twelve genotypes (2001MS7003, 2001MS7013, 2001 MS 7015, IESV 92084-DL, IESV 92168-DL, 2001 MS 7007, 2005 MI 5060, 2005 MI 5064, 2005 MI 5065, 2005 MI 5066, 2005 MI 5069, and Melkam) had above average grain filling rate (66.29 kg/day/ha). The highest grain filling rate across locations was by genotype IESV 92084-DL (77.59 kg/day/ha). This genotype was among the high yielder genotypes over locations and is preferred by its time consumption to fill the grain (Table 6). The average grain filling rates of genotypes were 54.63 kg/day/ha at Errer, 74.91 kg/day/ha at Kobo, 46.17 kg/day/ha at Mieso and 89.45 kg/day/ha at Shewa Robit (Table 8). The variation among means of grain filling rate of genotypes in the four locations was wide. The grand mean grain filling rate of locations was 66.29 kg/day/ha, Shewa Robit and Kobo were the two locations that had faster grain filling rate than the rest two locations. At Shewa Robit, genotypes filled their grains at a faster rate than the genotypes in the other locations. At Mieaso, grain filling rate was the poorest of all the locations. As discussed above, plant height, stand count at harvest and grain filling rate of the tested genotypes was not affected by the interaction effect. Hence, for these traitsgenotypes are compared from their mean potential of single location. The mean plant height, stand count at harvest and grain filling rate of genotypes tested at four sorghum growing locations are presented on table 7. Sorghum leaves are used as feed for animals, and the stalk as housing and fencing material, and as energy source. Due to this reason, sorghum farmers in the lowlands of Ethiopia set plant height (as component of biomass) as one of the selection criteria for sorghum varieties. As a result, for better adoption of varieties, this parameter should be considered as one of the major selection criteria during evaluation. Differences in the plant height of sorghum genotypes were observed at Errer and Kobo only (Table 7). At Errer, genotypes 2005 MI 5065 (2.08 m), 2005 MI 5060 (1.94 m), IESV 92168-DL (1.93 m) and 2005 MI 5075 (1.93 m) were the tallest genotypes. The tallest genotypes at Kobo were 2005 MI 5075 (1.97 m), 2005 MI 5060 (1.96 m), 2001 MS 7037 (1.95 m) and 2005MI 5069 (1.88 m). The standard check variety (Melkam) was found to be the shortest of all the genotypes with height of 1.44 m and 1.5 m at Errer and at Kobo, respectively (Appendix 2 and 3). 32 The mean plant height of all the genotypes at the tested locations was 183.00 m. Average height of genotypes at Errer (179.21 m), Kobo (181.35 m) and Shewa Robit (183.87 m) were not statistically different. The highest mean plant height of the genotypes was observed at Mieso, it is significantly higher than that of Errer and Kobo, but statistically similar from the mean plant height of genotypes at Shewa Robit (Table 7). Statistically there was no significant difference among the genotypes for the average number of days that the tested genotypes reached physiological maturity after flowering (grain filling period) (Table 7). A genotype that has longer reproductive stage would have higher grain weight and number of seeds per head. Moreover, due to the longer grain-filling period and increased vegetative growth late maturity hybrids tend to yield higher than shorter season sorghum hybrids (Baumhardt et al. 2005). Unfortunately, it was difficult to confirm these findings in the present study as differences were not significant. 33 Table 7 Mean plant height, stand count at harvest and grain filling period of twenty two early maturing sorghum genotypes tested at Errer, Kobo, Mieaso and Shewa Robit during 2014 main cropping season. Entry Genotypes PH 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 2001 MS 7003 2001 MS 7013 2001 MS 7015 2001 MS 7037 IESV 92084-DL IESV 92168-DL IESV 92199-DL IESV 92057-DL IESV 9027-DL 2001 MS 7007 2005 MI 5060 2005 MI 5064 2005 MI 5065 2005 MI 5066 2005 MI 5069 2005 MI 5070 2005 MI 5075 2005 MI 5079 2005 MI 5081 2005 MI 5082 ICSR 24005 Melkam Mean CV (%) bcd 174.33 164cde 170bcde 176.67bcd 182.33bcd 193.67ab 168.67bcde 189abc 160de 168bcde 194ab 187abcd 207.67a 180.33bcd 184.67abcd 183bcd 193ab 181.67bcd 174bcd 183bcd 183.33bcd 144.33e 179.21 7.92 Errer SCH 40.67 24.33 32.33 30.67 41.33 24.00 33.67 44.00 23.67 28.33 45.00 51.67 35.33 32.33 24.33 51.67 32.67 35.33 21.67 37.33 28.67 25.00 33.82 37.58 GFP 55.00 55.00 55.67 55.67 54.67 55.33 53.67 56.33 56.00 55.33 54.67 55.33 55.33 56.67 55.67 53.67 55.67 55.00 54.67 56.33 56.33 56.00 55.63 3.46 PH ab 187.33 175.67ab 180.67ab 195.33a 160.33bc 181.67ab 172.67abc 178.67ab 170.33abc 186.33ab 195.67a 184ab 187.33ab 187.33ab 188.33a 183.67ab 196.67a 187ab 178.67ab 180.67ab 181.33ab 150c 181.35 7.69 Experimental Locations Kobo Mieso SCH GFP PH SCH 44.00 36.67 41.00 34.67 44.67 38.67 44.33 49.33 39.00 26.33 50.33 39.67 35.67 37.67 41.00 38.00 33.33 33.67 42.00 31.00 29.33 41.33 38.71 22.21 35.00 35.00 36.00 35.67 33.67 36.33 36.67 38.67 37.67 37.33 34.00 38.33 38.67 36.33 36.67 34.33 37.33 36.33 34.00 34.67 39.67 37.67 36.36 6.29 165.00 170.00 186.67 190.00 185.00 198.33 193.33 185.00 170.00 170.00 206.67 200.00 200.00 198.33 195.00 195.00 193.33 186.67 188.33 188.33 191.67 170.00 187.58 9.13 54.67 56.33 65.00 54.33 55.00 55.33 60.33 57.67 49.33 55.33 68.67 56.33 59.00 53.67 50.33 59.67 51.67 61.67 54.33 57.67 52.67 64.00 56.95 12.41 GFP 51.00 50.67 50.00 49.00 50.00 50.00 50.67 50.33 51.00 49.67 53.33 54.67 48.00 49.67 47.67 48.33 49.33 50.00 50.67 49.33 50.33 52.33 50.27 6.21 Shewa Robit PH SCH GFP 181.47 188.80 181.60 179.40 191.80 205.73 178.87 187.67 186.73 170.33 167.60 179.27 197.00 198.03 177.73 189.40 185.07 183.27 174.67 179.40 187.73 173.67 183.87 7.35 84.33a 66bc 69abc 73abc 77.33ab 66.33bc 69.67abc 71.33abc 64.33bc 71.33abc 72.33abc 59cd 65.33bc 73.67abc 39.67e 68.33abc 66.67bc 61bc 64bc 72abc 45.33de 76.67abc 67.12 13.35 52.67 53.67 54.33 50.00 54.00 56.33 55.00 56.33 55.67 59.00 53.67 56.33 59.33 51.33 48.67 56.00 55.67 54.33 51.33 56.33 55.33 56.67 54.64 8.56 PH = plant height, SCH = stand count at harvest, GFR = grain filling rate. Means within the same column following by the same letter are not significantly different at 5 %. 34 Grain filling periods of genotypes at Errer (55.63 days) and Shewa Robit (54.64 days) were statistically equal. At these two locations grain filling period of sorghum genotypes was large as compared to the rest locations. At Mieaso the average period of genotypes to fill their grain was 50.27 days, this makes the grain filling period of genotypes at Mieso is faster than Errer and Shewa Robit and late from that of Kobo. As compared to the three locations the smallest period of time that genotypes required to fill their grain was observed at Kobo, it was 36.36 days. This variation might be due to the differences of locations in the amount of rain fall they obtained (Table 8). Table 8 Means for days to emergence, flowering and maturity, plant height, stand count at harvest, grain yield, grain filling period and grain filling rate of twenty two early maturing genotypes at Errer, Kobo, Mieso and Shewa Robit. Location Traits DE DF DM PH SCH GY GFP GFR Errer 8.00b 80.65b 136.02a 179.02b 34.33d 3.02b 55.36a 55.26c Kobo 6.00c 82.89a 119.26d 181.35b 38.71c 2.72c 36.36c 74.91b Mieaso 5.45d 73.36d 123.64c 187.58a 56.96b 2.32c 50.27b 46.17d 77.09c 131.73b 183.87ab 67.12a 4.87a 54.64a 89.45a Shewa Robit 10.20a Mean 7.41 70.504 127.66 183.00 49.15 3.23 49.16 66.29 CV (%) 6.14 4.68 2.70 8.07 19.45 12.70 6.47 14.13 DE = days to emergence, DF = days to flowering. DM = days to maturity, PH = Plant height, SCH = stand count at harvest, GY = grain yield, GFP = grain filling period and GFR = grain filling rate. Means of locations within the same column following by the same letter are not significantly different at 5 %. The trial environment, agronomic practice and sorghum genotypes significantly affected the required days for flowering and maturity Sally (2012). In the present study the effects of location were very highly significant for both genotypes flowering and maturity day. Each of the tested locations had significantly different among each other (Table 8). The mean days of flowering that genotypes required are significantly early at Mieso (73 days) and late at Kobo (83 days). Genotypes mean flowering date was 81 days at Errer and 77 days at Shewa Robit. 35 The mean maturity days of locations were not statistically similar. At Mieaso (124 days) the required mean genotypes maturity days were significantly early than mean days of genotypes maturity day at Shewa Robit (132 days) and Errer (136 days), but was significantly late from that of at Kobo (119 days). Compared to the overall locations flowering and maturity date, it was only at Errer and Kobo that had above the mean of the four locations flowering date (79 days). The overall maturity date of locations was 128 days. At Kobo and Mieaso the mean maturity days were lower than the grand mean (Table 8). 4.3. Genotype x Environment Interaction Analysis of Variance 4.3.1. Genotype x Environment Interaction Analysis of Variance by Eberhart and Russel's Model Genotype x environment interaction ANOVA of joint linear regression model is used for estimation and partitioning of GE interaction in to components. The analysis of Variance by Eberhart and Russel's Model of early maturing sorghum genotypes on mean grain yield (ton/ha) tested at four locations is presented in table 9. Eberhart and Russell (1966) procedure involves the use of joint linear regression where the yield of each genotype is regressed on the environmental mean yield. In this model the SS due to environments and GEI are partitioned into environments (linear), GE (linear) and deviations from regression (pooled deviation over all the genotypes). The genotype regressions term was tested for significance using an F-ratio by taking the deviations from regressions mean square as the error term. The deviations from regressions mean square were tested for significance using the error term for overall GEI in the ANOVA. The result of Eberhart and Russell’s ANOVA revealed non-significant (P≤ 0.05) difference among the genotypes for grain yield indicating the yield performance of genotypes was similar. The GE (linear) interaction was not significant. Thus, the GE interaction was nonlinear type and shows the nonexistence of genetic differences among genotypes for their response to varying locations, which is in agreement with earlier findings of Kenga et al. (2003), Wedajo (2014), and Fekadu et al. (2009). Pooled deviations were highly significant against pooled error. 36 Table 9 Analysis of Variance by Eberhart and Russel's Model of early maturing sorghum genotypes on mean grain yield (ton/ha) tested at four locations. Source of Variation Df Sum squares Mean Squares Total 87 113.2829 Genotype 21 7.7600 0.3695ns Loc. + (Gen. x Loc.) 66 105.5229 1.599** Location (Linear) 1 84.0310 84.031** Genotype x Location (Linear) 21 6.9206 0.3296ns Pooled Deviation 44 14.5713 0.3312** Genotype 1 2 0.3371 0.169ns Genotype 2 2 1.5867 0.793** Genotype 3 2 0.0538 0.027ns Genotype 4 2 0.1634 0.082ns Genotype 5 2 0.1200 0.06ns Genotype 6 2 1.0382 0.519** Genotype 7 2 0.3339 0.167ns Genotype 8 2 0.6477 0.324** Genotype 9 2 0.9680 0.484** Genotype 10 2 0.3151 0.158ns Genotype 11 2 0.3954 0.198ns Genotype 12 2 0.4511 0.226* Genotype 13 2 0.0972 0.049ns Genotype 14 2 0.1768 0.088ns Genotype 15 2 0.1726 0.086ns Genotype 16 2 1.7507 0.875** Genotype 17 2 0.4997 0.250* Genotype 18 2 0.3460 0.173ns Genotype 19 2 0.5103 0.255* Genotype 20 2 1.7500 0.875** Genotype 21 2 0.3072 0.154ns Genotype 22 2 2.5505 1.275** Pooled Error 176 11.5122 0.0654 ** = highly significant (P<0.001), * = significant (P<0.0=01) and ns = non-significant (P>0.05). In addition, only 32.176 % of the GEI sum of squares accounted by regression sum of square, and the remaining 67.824 % was accounted for the SS of the regression deviation. This indicates that the largest proportion of the interaction component of variation was explained 37 by the deviation from regression. Hence, according to Khan et al. (1988) and Ashraf et al. (2001), such differences in stability were due to deviation from linear regression only. This means the variation in the yield performance of genotypes are entirely unpredictable in nature. 4.3.2. Genotype x Environment Interaction Analysis of Variance by AMMI Model The combined AMMI ANOVA of the twenty two early maturing sorghum genotypes over four locations for grain yield (ton/ha) is presented in Table 10. The ANOVA indicated very highly significant differences (p<0.01) for treatments (environments, genotypes and GEI). Table 10 AMMI analysis of variance for grain yield (ton/ha) of early maturing sorghum genotypes tested at four locations during 2014 main cropping season. Source Total Treatments Genotypes Location Block GEI IPCA 1 IPCA 2 Residuals Error DF = degree DF SS % Total 263 374.4 87 339.8 90.75 21 23.3 3 252.1 8 6.2 63 64.5 23 32.0 21 18.7 19 13.7 168 28.4 7.59 of freedom, SS =sum of Sum of squares explained % % % Treatment GxL Cumulative 6.86 74.19 18.98 49.61 28.99 21.24 squares, MS = mean of MS 1.424 3.906*** 1.109 84.031 0.771 1.023 49.61 1.392*** 78.60 0.892*** 99.84 0.722*** 0.169 squares and *** = vary highly significant (P<0.0001) The total variation explained (%) was 90.75 % for treatment and 7.59 % for error. The greater contribution of the treatment than the error indicates the reliability of this multi-location experiment. The treatment variation was largely due to among locations variation (74.19 %), genotype and GEI accounted 6.86 % and 18.98 % for the treatment variation, respectively. As mentioned earlier, the high percentage of the location is an indication that the major factor that influence yield performance of sorghum in Ethiopia is the environment. In the AMMI ANOVA the GEI was further partitioned by PCA. The Gollob F-test used to measure significant of the GEI components. The number of PCA axis to be retained is determines by 38 testing the mean square of each axis with the estimate of residual through the F-statistics. The result of ANOVA showed that the first two IPCA are significant at 0.001 probability level, this result suggests the inclusion of the first two interactions PCA axes in the model. Hence, the best fit AMMI model for this multi-location yield trial data was AMMI-2 (Table 10). In particular, the first IPCA captured 49.61 % of the total interaction sum of squares while the second IPCA explained 28.99 % of the interaction sum of squares. Gauch and Zobel (1996) and Yan et al. (2002) also suggested that the most accurate model for AMMI can be predicted by using the first two IPCAs. In the present study the first two IPCAs accounted for a total of 78.60 % of the interaction with 44 of the corresponding degrees of freedoms. This indicates that the GEI of the twenty two sorghum genotypes with four locations was sufficiently predicted by the first two principal components axes and therefore, most information may well to graphically display in AMMI1 and AMMI2 biplot. However, due to the fact that as the result of the ANOVA indicates the residual of the interaction was also significant; indicating, the presence of unpredictable source of variation for the sum of squares of the interaction. Therefore, that is impossible to express the GEI of the twenty two sorghum genotypes tested at four locations using the first two principal components axes and no need of going further to graphically display in AMMI1 and AMMI2 biplot. 4.4. Stability Analysis 4.4.1. Stability Analysis by Eberhart and Russel's Model The stability parameters of Eberhart and Russell’s (1966) model for the yield of early maturing sorghum genotypes tested at four locations is presented in table 11. According to this model the genotype’s performance is expressed in terms of three parameters, mean yield, regression coefficient and the deviation from the regression. Therefore, a stable genotype is one with high mean yield, bi=1, and S2di not significantly different from zero. 39 Table 11 Eberhart and Russell’s (1966) stability parameters of early maturing sorghum genotypes tested at four locations. Genotypes Designation Genotypes 𝑏𝑖 𝑆𝑑2𝑖 GY (ton/ha) GY Rank 1 Code 2 3 4 5 6 2001 MS 7003 2001 MS 7013 2001 MS 7015 2001 MS 7037 IESV 92084-DL IESV 92168-DL 0.9456 1.0331 0.9754 0.9629 1.5200 1.5800 0.1031 0.7279** -0.039 0.0163 -0.005 0.4537** 3.34 3.33 3.3 2.57 3.71 3.33 8 9 12 22 1 9 7 8 IESV 92199-DL IESV 92057-DL 0.4188 0.9295 0.1016 0.2584** 2.88 3.12 19 15 9 10 IESV 9027-DL 2001 MS 7007 0.9663 1.3377 0.4186** 0.0921 2.86 3.47 20 5 11 12 2005 MI 5060 2005 MI 5064 0.8795 0.9718 0.1323 0.1601* 3.37 3.68 6 2 13 14 15 2005 MI 5065 2005 MI 5066 2005 MI 5069 1.4846 0.9501 0.7777 -0.0168 0.0230 0.0209 3.67 3.35 3.09 3 7 16 16 17 2005 MI 5070 2005 MI 5075 1.1498 1.1566 0.8099** 0.1844* 3.23 3.09 14 16 18 19 2005 MI 5079 2005 MI 5081 0.8263 0.5460 0.1076 0.1897* 3 2.67 18 21 20 21 2005 MI 5082 ICSR 24005 0.9471 0.6100 0.8096** 0.0882 3.25 3.31 13 11 22 Melkam 1.0311 1.2098** 3.49 4 The result of an individual genotypes deviation from linear regression (Table q) showed that genotype 2001 MS 7003, 2001 MS 7037, 2001 MS 7015, IESV 92084-DL, IESV 92199-DL, 2001 MS 7007, 2005 MI 5060, 2005 MI 5065, 2005 MI 5066, 2005 MI 5069, 2005 MI 5079 and ICSR 24005 had non-significant deviation from regression. The 𝑏𝑖 estimate of these genotypes ranged from 0.0895 to 1.5199, and the 𝑏𝑖 estimates of genotype 2001 MS 7003 (0.95), 2001 MS 7037 (0.96), 2001 MS 7015 (0.98), 2005 MI 5060 (0.89) and 2005 MI 5066 (0.95), are relatively near to unity. The average yield performance of genotype 2001 MS 40 7037, was below average (Table 11). Therefore, considering their above average mean grain 2 yield, 𝑏𝑖 value closest to unity and the𝑆𝑑𝑖 = 0, genotype 2001 MS 7003, 2001 MS 7015, 2005 MI 5060 and 2005 MI 5066 were the most stable genotypes based on Eberhart and Russell’s 2 model. In contrary, the 𝑆𝑑𝑖 value of genotype 2001 MS 7013, IESV 92168-DL, IESV 92057- DL, IESV 9027-DL, 2005 MI 5064, 2005 MI 5070, 2005 MI 5075, 2005 MI 5081, 2005 MI 5082 and Melkam were significantly different from zero (Table 11). Hence, these genotypes were unstable in a wide range of environments. Wachira et al. (2002) categorized genotypes adaptability to specific environments based on their estimate of 𝑏𝑖 as adaptable to high and low yielding environments. The 𝑏𝑖 values above one describe genotypes with higher sensitivity to environmental change (below average stability) and are suitable to high yielding environments, whereas 𝑏𝑖 below one provides a measurement of greater resistance to environmental change (above average stability), and are adaptable to low yielding environments. Based on this concept the present study indicates (Table 11), genotype 2001 MS 7013, IESV 92084-DL, IESV 92168-DL, 2001 MS 5007, 2005 MI 5065, 2005 MI 5070, 2005 MI 5075 and Melkam had 𝑏𝑖 value of greater than one and above mean performance. Therefore, these genotypes contributed a lot to the GEI and were suitable for favorable environments. In contrary, genotype 2001 MS 7037, IESV 92199-DL, IESV 92057-DL, IESV 9027-DL, 2005 MI 5064, 2005 MI 5069, 2005 MI 5079, 2005 MI 5081, 2005 MI 5082 and ICSR 24005 had 𝑏𝑖 value less than unity and these genotypes contributed less to the GEI. Hence, these genotypes are suitable for unfavorable environments (Table 11). 4.4.2. Yield Stability Using ASV In additive main effect and multiplicative interaction effect stability analysis (ASV) method, a genotype with least ASV score is the most stable across environments and the larger the ASV value, either negative or positive, the more specifically adapted a genotype is to certain environments (Purchase, 1997). Table 12 indicates ASV for each genotype and the ranks of the genotypes according to their AS values. 41 Table 12 IPCA1 and IPCA 2 scores; and ASV for the twenty two early maturing sorghum genotypes sorted on mean yield (ton/ha) evaluated at four locations during 2014 main cropping season. Genotypes Designation Genotypes GY (ton/ha) GY Rank IPCA 1 Score IPCA 2 Score ASV ASV Rank 1 Code 2 3 4 5 6 2001 MS 7003 2001 MS 7013 2001 MS 7015 2001 MS 7037 IESV 92084-DL IESV 92168-DL 3.34 3.33 3.3 2.57 3.71 3.33 8 9 12 22 1 9 -0.2281 -0.3431 -0.1032 -0.1547 -0.2701 -0.6682 -0.2507 -0.3776 -0.0934 -0.1755 0.6052 0.5449 0.4526 0.7288 0.1851 0.2951 0.8278 1.4387 9 14 3 6 15 22 7 8 IESV 92199-DL IESV 92057-DL 2.88 3.12 19 15 0.5577 0.3956 -0.4817 0.192 1.1850 0.7128 18 13 9 10 IESV 9027-DL 2001 MS 7007 2.86 3.47 20 5 -0.4632 -0.0808 -0.3279 0.5136 0.8990 0.4019 17 8 11 12 2005 MI 5060 2005 MI 5064 3.37 3.68 6 2 0.3676 -0.0996 0.0499 -0.231 0.6306 0.2236 11 4 13 14 15 2005 MI 5065 2005 MI 5066 2005 MI 5069 3.67 3.35 3.09 3 7 16 -0.2797 0.1857 -0.0611 0.4079 0.0915 -0.262 0.6443 0.3257 0.1730 12 7 2 16 17 2005 MI 5070 2005 MI 5075 3.23 3.09 14 16 0.5881 0.0135 0.4797 0.0516 1.2350 0.0257 20 1 18 19 2005 MI 5079 2005 MI 5081 3.00 2.67 18 21 -0.0749 0.2851 -0.3747 -0.2725 0.2684 0.5614 5 10 20 21 2005 MI 5082 ICSR 24005 3.25 3.31 13 11 0.676 0.4591 0.3282 -0.2773 1.2628 0.8614 21 16 3.49 3.23 4 -0.7018 -0.1401 1.2188 19 22 Melkam Mean The results showed that from the tested early maturing sorghum genotypes seventeen of them had ASV of below one. Accordingly 2005 MI 5075, 2005 MI 5069, 2001 MS 7015, 2005 MI 5064, 2005 MI 5079, 2001 MS 7073, 2005 MI 5066, 2001 MS 7007, 2001 MS 7003, 2005 MI 5081, 2005 MI 5060, 2005 MI 5065, IESV 92057-DL, 2001 MS 7013, IESV 92084-DL, ICSR 24005 and IESV9027-DL were relatively widely stable (Table 12). In contrary, due to 42 their large ASV genotype IESV 92199-DL, Melkam, 2005 MI 5070, 2005 MI 5082 and IESV 92168-DL were the most unstable genotypes (Table 12). The mean yield of genotypes is also considered for selection of genotypes as a high yielder and stable genotypes. From the selected widely stable early maturing genotypes the mean yield of ten genotypes are above the grand mean. Therefore, based on ASV, genotype 2001 MS 7015, 2005 MI 5064, 2005 MI 5066, 2001 MS 7007, 2001 MS 7003, 2005 MI 5060, 2005 MI 5065, 2001 MS 7013, IESV 92084-DL and ICSR 24005 are relatively high yielder and widely stable genotypes. The five most stable and high yielder early maturing sorghum genotypes on this model were genotype 2001 MS 7015, 2005 MI 5064, 2005 MI 5066, 2001 MS 7007 and 2001 MS 7003 (Table 12). 43 5. SUMMARY AND CONCLUSIONS A total of 22 EMSGs were evaluated at Errer (Errer Agricultural Research sub-center), Kobo (Sirinka Agricultural Research Center), Mieso (Sub-center for Melkasa Agricultural Research Center) and Shewa Robit (Debre Birhan Agricultural Research Center) during the 2014 main cropping season with the objectives of estimating the magnitude of GEI for grain yield and other traits and to determine stability effect on grain yield. The ANOVA for each location showed that the genotypes were significantly different for grain yield (ton/ha) and grain filling rate. In contrast, for each location, insignificant variation among genotypes was obtained for grain filling period. The variation among genotypes for days to emergence, days to flowering, days to maturity, plant height and stand count at harvest was different from location to location. Genotypic differences were significant for plant height, but non-significant for days to emergence, days to flowering, days to maturity and stand count at harvest at Errer. Genotypic differences were non-significant for days to emergence, days to flowering, days to maturity, plant height and stand count at harvest at Mieso. However, days to flowering, days to maturity and plant height at Kobo and days to emergence and stand count at harvest at Shewa Robit were found to be significant. The combined ANOVA across the four locations revealed significant differences among the sorghum genotypes for plant height (p≤ 0.001), stand count at harvest (p≤ 0.01) and grain filling period (p≤ 0.05). There was also highly significant difference among the tested locations for the entire measured parameters. The total variation in yield was attributed to 67.34 % to location, 6.24 % to genotype and 17.22 % to the interaction effects. The grand mean yield of genotypes across location was 3.23 ton/ha. The highest mean grain yield (4.87 ton/ha) was recorded at Shewa Robit followed by at Errer (3.22 ton/ha). ). From the tested genotypes genotype 2001 MS 7003, 2001 MS 7013, 2001 MS 7015, IESV 92084DL, IESV 92168-DL, 2001 MS 7007, 2005 MI 5060, 2005 MI 5064, 2005 MI 5065, 2005 MI 5066, 2005 MI 5082, ICSR 24005 and Melkam had mean yield above the grand mean. Genotype ICSR 24005 had maximum number of days to emerge and genotype 2005 MI 5064 had numerically minimum number of days to emerge. Genotypes emergence period was 44 minimum at Mieso and maximum at Shewa Robit. The Grain filling rate of genotypes was significantly fast at Shewa Robit and slow at Mieso. Due to insignificant effect of interaction on the performance of genotypes for days to flowering and maturity, plant height, stand count at harvest, and grain filling period, selection of genotypes for these traits could be carried out based on their genetic potential. The relative performance of genotypes for days to emergence, grain yield and grain filling rate was significantly affected by the varying environmental conditions. Hence, Eberhart and Russell’s joint regression model, AMMI model and ASV models were used for grain yield to identify superior, adaptable and relatively stable genotypes across location. Eberhart and Russell’s joint regression ANOVA showed that the performance of genotypes for grain yield was statistically similar. The GE (Linear) was insignificant and the pooled deviation was significant. The interaction sum square was accounted largely by the pooled deviation (67.82 %) and only 32.78 % by the GE (Linear). The mean square of pooled deviation of genotype 2001 MS 7013, IESV 92168-DL, IESV 92057-DL, IESV 9027-DL, 2005 MI 5064, 2005 MI 5070, 2005 MI 5075, 2005 MI 5081, 2005 MI 5082 and Melkam was significantly different from zero. The combined AMMI ANOVA showed lack of genotype differences and significant differences among genotypes and the presence of interaction effect. In this study including the IPCA residual both the first two IPCAs were significant. For the total variation the treatment variation accounted about 90.75 %, and for the treatment variation was attributed to genotype variation 6.86 %, location variation 74.18 % and interaction 18.98 %. In addition 78.60 % of the interaction effect was explained by the first two IPCAs. Based on Eberhart and Russell’s stability analysis, considering their above average mean 2 grain yield, 𝑏𝑖 value closest to unity and the𝑆𝑑𝑖 = 0, genotype 2001 MS 7003, 2001 MS 7015, 2005 MI 5060 and 2005 MI 5066 were the most stable genotypes. Genotype 2001 MS 7013, IESV 92084-DL, IESV 92168-DL, 2001 MS 5007, 2005 MI 5065, 2005 MI 5070, 2005 MI 5075 and Melkam had 𝑏𝑖 value of greater than one and above mean performance and are selected for favorable locations. Genotype 2001 MS 7037, IESV 92199-DL, IESV 92057-DL, IESV 9027-DL, 2005 MI 5064, 2005 MI 5069, 2005 MI 5079, 2005 MI 5081, 2005 MI 5082 45 and ICSR 24005 had 𝑏𝑖 value less than unity and these genotypes are suitable for unfavorable environments. Additive Main Effects and Multiplicative Interaction stability value (ASV) was one of the stability models to identify the stable genotype for this study. Accordingly, The five most stable and high yielder early maturing sorghum genotypes on this model were genotype 2001 MS 7015, 2005 MI 5064, 2005 MI 5066, 2001 MS 7007 and 2001 MS 7003. The results of genotype x environment interaction and stability analysis indicated that, both Eberhart and Russell’s stability analysis and ASV models identified threeearly maturing sorghum genotypes(2001 MS 7003, 2001 MS 7015 and 2005 MI5066), that had a high mean performance and high stability for yield. Therefore, genotype 2001 MS 7003, 2001 MS 7015 and 2005 MI5066 can be recommended as a candidate for releasing over a wide range of locations of the lowland Ethiopia. This study highlighted important points for future studies related to allocation of EMSGs to different growing conditions in the lowlands of Ethiopia. The sorghum growing dry lowland areas of Ethiopian were diverse and contributed largely to the changes of genotypes yield performance over locations. Therefore, further study on the GEI effects and stability of early maturing sorghum genotypes is needed in multi locations for a number of years and location to identify the interaction effect of genotypes and select stable genotypes. 46 6. REFERENCES Abubakar, L. and Bubuche, T. S. 2013. Genotype x environment interaction on yield and its component of sorghum (Sorghum bicolor(L.) Moench) genotypes of some selected states in North-Western Nigeria. International Journal of Current Agricultural Research, 1(6): 27-29. http://www.wrpjournals.com/journals/V/IJCAR Ahmed, M. E., Ibrahim, M. I., Mohammed, E. A. and Elshiekh, A. I. 2012.Evaluation of some local sorghum (Sorghum Bicolor(L.) Moench) Genotypes in Rain-Fed. International Journal of Plant Research, 2(1): 15-20. Akcura, M., Kaya, Y., Taner, S. and Ayranci, R. 2006. Parametric stability analyses for grain yield of durum wheat. Plant Soil Environ, 52(6): 254–261. Ali, S.S., Manzoor, Z., Awan, T.H. and Mehdi, S.S. 2006. Evaluation of performance and stability of sunflower genotypes against salinity stress. Journal of Animal and Plant Sciences, 16(2):47-51. Allard, R.W. and Bradshaw, A.D.1964. Implications of Genotype by environment interactions in applied plant breeding. Crop science, 4:503-508. Almeida, F.J.E., Tardin, F.D., Daher, R.F., Barbe, T.C., Paula, C.M., Cardoso, M.J. and Godinho, V.P.C. 2014. Stability and adaptability of grain sorghum hybrids in the off-season. Genetic and Molecular Research, DOI http://dx.doi.org/10.4238/. A head of print. Asfaw Adugna, Tesfaye Tesso, Erenso Degu, Taye Tadesse, Feyera Merga, Wasihun Legesse, Alemu Tirfessa, Haileselassie Kidane, Andualem Wole and Chemeda Daba. 2011. Genotype by environment interaction and yield stability analysis in finger millet (Elucine coracana L. Gaertn) in Ethiopia. American Journal of Plant Sciences, 2: 408-415. Asfaw Adugna. 2007. Assessment of yield stability in sorghum. African Crop Science Journal, 15 (2): 83 – 92. Asfaw Adugna. 2008. Assessment of yield stability in sorghum using univariate and multivariate statistical approaches. Hereditas, 145: 28-37. 47 Ashok, K., Belum, V. S. R., Hari, C.S., Charles, T. H., Pinnamaneni, S. R., Bhavanasi, R. and Pulluru, S. R. 2011. Recent advances in sorghum genetic enhancement research at ICRISAT. American Journal of Plant Sciences, 2: 589-600. Ashraf, M.A., Qureshi, S., Ghafoor, A. and Khan, N.A. 2001. Genotype by environment Interaction in wheat. Asian Journal of Biological Science, 1:356-357. Assefa, Y. and Staggenborg, S.A. 2010. Grain sorghum yield with hybrid advancement and changes in agronomic practices from 1957 through 2008. Agronomy Journal, 102: 703- 706. Assefa, Y.S., Staggenborg, A. andPrasad, P.V.V. 2010. Grain sorghum water requirement and responses to drought stress: A review. On-line. Crop Management doi: 10.1094/CM-201011109-01-RV. Baker, R.J. 1990. Crossover genotype by environmental interaction in spring wheat. pp. 42–51. In: Kang, M.S. (Ed.) Genotype by Environment Interaction and Plant Breeding. Louisiana State University Agricultural Center, Baton Rouge, Louisiana. Baumhardt, R.L., Tolk, J.A. and Winter, S.R. 2005. Seeding practices and cultivar maturity effects on simulated dryland grain sorghum yield." Agronomy Journal, 97.3: 935-42. Becker, H.C. and Leon, J. 1988. Stability analysis in plant breeding. Plant Breeding, 101:1–23. Becker, H.C. 1981. Correlations among some statistical measures of phenotypic stability. Euphytica, 30:832-840. Chahal, G.S. and Gosal, S.S. 2002. Principles and procedures of plant breeding: biotechnologicaland conventional approaches. Narosa PublishingHouse. New Delhi, India. CSA (Central Statistical Agency). 2014. Agricultural Sample Survey, Report on Area and Production of Crops for Private Peasant Holdings, Maher Season, Addis Ababa, Ethiopia. Comstock, R.E. and Moll, R.H. 1963. Genotype by environment interactions. Statistical Genetics and Plant Breeding, 164-196. 48 Cornelius, P.L., Crossa, J. and Seyedsadr, M.S. 1996. Statistical tests and estimates of multiplicative models for GEI. pp. 199–234. In: Kang, M.S. and Gauch, H.G. (eds.) Genotype by Environment Interaction. CRC Press, Boca Raton, Florida. Crossa, J., Gauch, H.G. and Zobel, R.W. 1990. Additive Main Effects and Multiplicative Interaction analysis of two international maize cultivar trials. Crop Science, 30:493-500. Dagnachew Lule, Kassahun Tesfaye, and Girma Mengistu. 2014. Genotype by environment interaction and grain yield stability analysis for advanced triticale (x. triticosecale wittmack) genotypes in western Oromia, Ethiopia. Ethiopia Journal Science, 37(1):63–68. DeWet, J.M.J. 1978. Systematics and evolution of Sorghum sect. bicolor (Gramineae). American Journal of Botany, 65: 477–484. Dicko, M.H., Gruppen, H., Traore, A.S., Alphons, G.J., Voragen, A.G.J. and Berkel, W.J.H.van. 2006. Sorghum grain as human food in Africa: Relevance of content of starch and amylase activities. African Journal of Biotechnology, 5: 384-395. Dillon, S.L., Lawrence, P.K., Henry, R.J., Ross, L., Price, H.J., Johnston, J.S., (2004). Sorghum laxiflorumand S. macrospermum, the Australian native species most closely related to the cultivated S. Bicolor based on ITS1 and NDHF sequence analysis of 25 Sorghum species. Plant Syst. Evol. 249, 233-246. Dillon, S.L., Frances, M.S., Robert, J.H., Giovanni, C., Liz, I. and Slade, L.L. 2007. "Domestication to Crop Improvement: Genetic Resources for Sorghum and Saccharum (Andropogoneae)." Annals of Botany.5: 975-89. Doggett, H., 1976. Sorghum. In: Simmonds N.W. (Ed.), Crop Evolution, pp. 112–117. Longman. Domitruk, D.R., Duggah, B.L. and Fowler, D.B. 2001. Genotype by environment interaction of no-till winter wheat in Western Canada. Canadian Journal of Plant Science, 81:7-16. Ebdon, J.S. and Gauch, H.G. 2002. Additive Main Effect and Multiplicative Interaction analysis of national turf grass performance trials: Interpretation of genotype by environment interaction. Crop Science, 42:489–496. 49 Eberhart, S.A. and Russel, W.A. 1966. Stability parameters for comparing varieties. Crop Science, 6:36-40. Fahri, A. 2012. Yield stability of some Turkish winter wheat (Triticum aestivum L.) genotypes in the western transitional Zone of Turkey. Turkish Journal of Field Crops, 17(2): 129-134. Faisal. E.A. and Aisha. O.A.H. (2011). Genotype x seed production environment interaction on the performance of sorghum (Sorghum bicolor [L.] Moench) under irrigation. Agricultural Biology Journal. North America, 2(4):2151-7517. Falconer, D.S. 1952. The problem of environment and selection. American Nature. 86: 293-298. Fekadu Gurmu, Hussein Mohamme and Getinet Alemaw. 2009. Genotype by environment Interactions and stability of soybean for grain yield and nutrition quality. African Crop Science Journal, 17(2):87 – 99. Fernandez, G.C.J. 1991. Analysis of genotype by environment interaction by stability estimates. Horticultural Science, 26(8): 947-950. Finley, W.K. and Wilkinson, G.N. 1963. The analysis of adaptation in a plant breeding program. Australian Journal of Agricultural Research, 14: 742-754. Firew Mekbib., 2009. Farmers’ breeding of sorghum in the centre of diversity, Ethiopia: I. Socio-ecotype differentiation, varietal mixture and selection efficiency. Maydica 54: 25-37. Flores, F., Moreno, M.T. and Cubero, J.I. 1998. A comparison of univariate and multivariate methods to analyze genotype by environment interaction. Field Crops Research, 56: 271-286. Fransis, T.R. and Kannenberg, L.W. 1978. Yield stability studies in short-season maize. I. A descriptive method for grouping genotypes. Canadian Journal of Plant Science, 58: 1029–1034. Gauch, H.G. and Zobel, R.W. 1996. Additive Main Effect and Multiplicative Interaction analysis of yield trials. pp 85-122. In: Kang, M.S. and Gauch, H.G. (Eds.), Genotype by environment interaction. CRC Press, Boca Raton, FL. Gauch, H.G. and Zobel, R.W. 1997. Identifying mega-environments and targeting genotypes. Crop science, 37(2): 311-326. 50 Gauch, H.G. 1988. Model selection and validation for yield trials with interaction. Biometrics, 44: 705-715. Ghazy, M.M.F., Shadia, M.S. and Magda, N. (2012). Stability analysis and genotype x environment interactions for forage sorghum hybrids (Sorghum bicolor(L.) Moench). Journal of Agricultural Research, 38(1):142-153. Gebremedhin Welu. 2015. Adaptation of food barley (Hordeum Vulgare L.) genotypes. Journal of Agricultural Sciences, 60 (2): 227-235. Geremew, Gemechu., Asfaw Adugna., Taye Tadese, Tsefaye Teferra., Ketema Belete., and Hilemichael, Habte. 2004. Development of sorghum varieties and hybrids for dryland areas of Ethiopia. Uganda Journal of Agricultural Science,9:594-605. Gomez, K.A. and Gomez, A.A. 1984. Statistical Procedures for Agricultural research. 2nded. John Wiley and Sons. Inc., New York, USA. 95-109. Hagos Tadesse and Fetien Abay. 2011. Additive Main Effects and Multiplicative Interactions analysis of yield performance of sesame genotypes across environments in Northern Ethiopia. Journal of the drylands, 4(1): 259-266. Hassan, Z., Ezatollah, F., Sayed, H.S. and Rahmatollah, K. 2012. Evaluation of genotype by environment interaction in chickpea using measures of stability from Additive Main Effect and Multiplicative Interaction model. Annals of Biological Research, 3 (7): 3126-3136. Human, S., Andreani, S. and Indriatama, W.M. 2011. Stability Test For Sorghum Mutant Lines Derived From Induced Mutations with Gamma-Ray Irradiation. Atom Indonesia, 37 (3): 102106. IBPGR and ICRISAT (International Board of Plant Genetic Resources and International Crop Research Institute for Semi-Arid Tropics). 1993. Descriptors for sorghum [Sorghum bicolor (L.) Moench]. IBPGR, Rome, Italy and ICRISAT, Patancheru, India 1-26. ICRISAT (International Crop Research Institute for Semi-Arid Tropics). 2005. Sorghum report. http://www.icrisat.org/text/research/grep/homepage/sorghum/sorghumhomepage.htm Patancheru, India. Online. 51 Jipan, S. 2013. Genotype by environment interaction and yield stability analysis of quality protein maize genotypes in Terai region of Nepal. Journal of Applied Science and Biotechnology, 1(2): 74-78. Jones, O.R., and Johnson. G.L. 1991. Row width and plant density effects on Texas high plains sorghum. Journal ofAgricultural production, 4:613–619. Kang, M.S. and Gauch, H.G. 1996. Genotype by Environment Interaction. CRC Press, Boca Raton, 416. Kang, M.S.1998. Using genotype by environment interaction for crop cultivar development. Advances in Agronomy, 62: 199-252. Kang, M.S. Aggarwal, V.D. and Chirwa, R.M. 2006. Adaptability and stability of bean cultivars as determined via yield-stability statistic and GGE biplot analysis. Journal of Crop Improvement, 15:97-120. Kebede, Y. (1991). The role of Ethiopian sorghum germplasm resources in the national breeding program. In: J.M., Engels, J. G. Hawke and M. Worede, (Eds.), Plant Genetic Resources of Ethiopia. Cambridge University Press, Cambridge, 315-322 pp. Kempton, R. 1984. The use of biplot in interpreting variety by environment interactions. Journal of Agricultural Science, 103, 123-135. Kenga, R., Alabi, S. and Gupta, O. 2003. Yield stability of sorghum hybrids and parental lines. African Crop Science Journal, 11(2): 65-73. Khan, M.I., Khan, N.A. and Khan, A.S. 1988. Evaluation of different sorghum genotypes for stability in yield performance. Pakistan Journal of Agricultural Research, 10: 237-243. Lazarides, M., Hacker, J.B. and Andrew, M.H., (1991). Taxonomy, cytology and ecology of indigenous Australian sorghums (Sorghum Moench: Andropogonae: Poaceae). Australian Botany, 4, 591-635. Lin, C.S., Bains, M.R. and Lefkovitch, L.P. 1986. Stability Analysis. Crop Science, 26:894-900. 52 Maposa, D., Mudimu, E. and Ngwenya, O. A. 2010. Multivariate analysis of variance (MANOVA) of the performance of sorghum lines in different agro-ecological regions of Zimbabwe. African Journal of Agricultural Research, 5(3): 196-203. Mahnaz, R., Ezatollah, F. and Mohammad, M. J. 2013. Additive Main Effect and Multiplicative Interaction Analysis of phenotypic stability in chickpea genotypes over stress and non-stress environments. International Journal of Agriculture and Crop Sciences, 5(36):253-260. Mesfin Abate, Firew Mekbib, Temam Hussien, Wondimu Bayu, and Fasil Reda. 2014. Assessment of genetic diversity in sorghum (Sorghum bicolor (L.) Moench) for reactions to Striga hermonthica (Del.) Benth.Australian Journal of Crop Science, 8(8):1248-1256. Molla Fentie, Alemayehu Assefa and Ketema Belete. 2013. AMMI Analysis of Yield performance and stability of finger millet genotypes across different environments. World Journal of Agricultural Sciences, 9 (3): 231-237. Muez Mehari, Sentayehu Alamerew, Berhane Lakew, Haddis Yirga and Mizan Tesfay. 2014. Parametric stability analysis of malt barley genotypes for grain yield in Tigray, Ethiopia. World Journal of Agricultural Sciences, 10 (5): 210-215. Oliveira, E., Freitas, J. and Jesus, O. 2014.Additive Main Effect and Multiplicative Interaction analysis of the adaptability and yield stability of yellow passion fruit varieties. Agricultural Science, 71:139-145. Perkins, J.M. and Jinks, J.L. 1968. Environmental and genotype by environmental components of variability. III. Multiple lines and crosses. Heredity 23: 339-356. Pinthus, M.J. 1973. Estimate of genotypic value: A proposed method. Euphytica, 22: 121- 123. Poehlman, J.M. and Sleper, D.A. 1995. Breeding Field Crops, 4th eds. Oxford and IBM pub. Co. New Delhi, India. 494. Prasad,P. V. V., Pisipati, S. R., Mutava, R. N. and Tuinstra, M. R. 2008. Sensitivity of grain sorghum to high temperature stress during reproductive development. Crop Science of America, 48:1911–1917. 53 Prasad, P.V.V. and Staggenborg, S.A. 2009. Growth and production of sorghum and millets. In soils, plant growth and crop production –Volume II. In: Encyclopedia of Life Support Systems, Eolss Publishers, Oxford, UK. http:// www.eolss.net. Access data. Purchase, J. L., Hatting, H. and Van Deventer, C.S. 2000. Genotype by environment interaction of winter wheat (Triticum aestivum L.) in South Africa Journal of Plant and Soil Sciences, 17: 101-107. Purchase, J.L. 1997. Parametric analysis to describe genotype by environment interaction and stability in winter wheat. PhD. thesis. Department of Agronomy, Faculty of Agriculture, University of the Orange Free State, Bloemfonten, South Africa. Rasul, I., Zulkiffal, M., Anwar, J., Khan, S.B., Hussain, M. and Riaz, D. 2006. Grain yield stability of wheat genotypes under different environment in Punjab. Journal of Agriculture and Social Sciences, 2(4): 222-224. Ryan. J.G. and Spencer D.C. 2001. Future challenges and opportunities for agricultural development in the Semi-arid Tropics. Patanchura 502 324, Andhra Paradesh, India. ICRISAT.pp 83. Sabaghnia, N., Dehghani, H. and Sabaghpour, S.H. 2006. Non-parametric methods for interpreting genotype by environment interaction of lentil genotypes. Crop Science Journal, 46: 1100-1106. Sanni, K.A., Ariyo, O.J., Ojo, D.K., Gregorio, G., Somado, E.A., Sanchez, I., Sie, M., Futakuchi, K., Ogunabayo, S.A., Guel, R.G. and Wopereis, M.C.S. 2009. Additive Main Effects and Multiplicative Interaction analysis of grain yield performance in rice genotypes across environments. Asian Journal of Plant Science, 8(1):48-53. Sayed, S.R., Ezatollah, F. and Ahmad, A.M. 2015. Additive main effects and multiplicative interaction analysis of yield performance in canola (Brassica napus L.) across different environments. Journal of Biodiversity and Environmental Sciences, 6(3): 134-140. 54 Sewagegne Tariku, Taddesse Lakew, Mulugeta Bitew and Mitiku Asfaw. 2013. Genotype by environment interaction and grain yield stability analysis of rice (Oryza sativa L.) genotypes evaluated in north western Ethiopia. Net Journal of Agricultural Science. 1(1): 10-16. Shrestha, S.P., Asch, F., Dusserre, J., Ramanantsoanirine, A. and Brueck, H. 2012. Climate effects on yield components as affected by genotypic responses to variable environmental conditions in upland rice systems at different altitudes. Field Crops Research, 134:216-228. Shrestha, J. 2013. Genotype by environment interaction and yield stability analysis of quality protein maize genotypes in Terai region of Nepal.International Journal of Applied Science Biotechnolgy,1(2): 74-78. Shukla, G.K. 1972. Some statistical aspects of partitioning genotype by environmental components of the variability. Heredity 29: 237-245. Singh, M., Ceccarelli, S. and Grando, S. 1999. Genotype by environment interaction of crossover type. Theoretical and Applied Genetics, 99, 988–995. Sneller, C.H., Kilgore Norquest, L. and Dombek, D. 1997. Repeatability of yield stability statistics in soybean. Crop Science, 37:383-390. Srinivasa, RAO P., Sanjana Reddy, P., Abhishek, R., Belum, V. R. and Sanjeev, P.2011. Application GGE biplot and AMMI model to evaluate sweet sorghum (Sorghum bicolor) hybrids for genotype × environment interaction and seasonal adaptation. Indian Journal of Agricultural Sciences, 81 (5): 438–44. Sun, Y., Chen, S.Q., Liang, G.H., (1994). Induction of autopolyploid plants of Sorghum bicolor. Cytology 59, 109-114. Sunday, C.O.M., Omolayo, J.A. and Raphael, I.A. 2013. Assessment of groundnut performance in different environments using Additive Main Effects and Multiplicative Interaction model. Canadian Journal of Plant Breeding, 1(2): 60-66. Tekle Yoseph and Zemach Sorsa. Evaluation of sorghum (Sorghum bicolor (L.) Moench) varieties for yield and yield components at Kako, Southern Ethiopia.Journal of Plant Sciences. 2(4): 129-133. 55 Tarakanovas, P. and Ruzgaz, V. 2006. Additive Main effects and Multiplicative Interaction analysis of grain yield of wheat varieties in Lithuania. Agronomy research, 4(1): 91-98. Teshome, A., Patterson, D., Torrance, J. K. and Arnason, J.T. (2007). Changes of Sorghum bicolor landrace diversity and farmers’ selection criteria over space and time, Ethiopia. Genetic resource and crop evolution. 54:1219–1233. Thangavel, P., Anandan, A. and Eswaran, R. 2011. Additive Main effects and Multiplicative Interaction analysis to comprehend genotype by environment interactions in rain fed grown mung bean (Vigna radiata L.). Australia Journal of Crop Science, 5(13):1767-1775. Thillainathan, M. and Fernandez, G.C.J. 2001. SAS applications for Tai's stability analysis and Additive Main effects and Multiplicative Interaction model in genotype by environment interactions effects. Journal of Heredity, 92 (4): 367-371. Vanderlip, R.L. and Reeves, H.E. 1972. Growth stages of sorghum [Sorghum Bicolor (L.) Moench]. Agronomy Journal, 64(1): 13-16. Vange, T., Ango, I. and Nache, A.V. 2014. Stability analysis of six improved sorghum genotypes across four environments in the Southern Guinea Savanna of Nigeria. Internationals Journal of Advances in Agricultural Science and Technology, 2 (2): 01-14. Vavilov, N.I. (1951). The origin, variation, immunity and breeding of cultivated plants. Chron. Bot., 13: 1-366. Wachira, F., Wilson, N.G., Omolo, J. and Mamati, G. 2002. Genotype x environment interactions for tea yields. Euphytica, 127: 289-296. Wedajo Gebre. 2014. Evaluation of pearl millet (Pennisetum glaucum L.) Genotypes for yield and yield stability in South Omo and West Hararghe. Journal of Biology, Agriculture and Healthcare.www.iiste.org. 4(8): 2224-3208. Wricke, G. 1962. Method of understanding the biological diversity in field research. Pfianzenzuchtg, 47: 92-146. 56 Yan, W. 2002. Singular value partitioning in biplot analysis of multi-environment trial data. Agronomy Journal, 94: 990-996. Yan, W. and Tinker, N.A. 2005. An integrated biplot analysis system for displaying, interpreting and exploring genotype by environment interactions. Crop Science, 45: 1004–16. Yan,W. and Hunt, L.A. 2002. Biplot analysis of multi-environment trial data. In: Quantitative Genetics, Genomics and Plant Breeding. Kang, M.S., (Ed.), CABI Publishing. Yonas Belete. 2014. Stability analysis of performance of hundred beans weight of Arabica coffee genotypes across different environments. Journal of Agricultural and Veterinary Science, 2(5):99-106. Yuksel, K., Palta, C. and Taner, S. 2002. Additive Main Effects and Multiplicative Interactions analysis of yield performances in bread wheat genotypes across environments. Turk Journal of Agriculture, 26:275–279. Zahra, R. Reza, H. and Hadi. P.A. 2013. Seed germination and seedling growth of three sorghum (Sorghum bicolor L.) genotypes as affected by low temperatures. International Journal of Farming and Allied Sciences,2(20):851-856. Zobel, W.R., Wright, M.J. and Gauch, H.G. 1988. Statistical analysis of a yield trial. Agronomy Journal, 80:388-393. 57 58 7. APPENDIX Appendix 1. Mean value of yield (ton/ha), phenological traitsand grain filling rate of early maturing sorghum genotypes for the data collected at Errer during 2014 main cropping season. Entry 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Genotypes 2001 MS 7003 2001 MS 7013 2001 MS 7015 2001 MS 7037 IESV 92084-DL IESV 92168-DL IESV 92199-DL IESV 92057-DL IESV 9027-DL 2001 MS 7007 2005 MI 5060 2005 MI 5064 2005 MI 5065 2005 MI 5066 2005 MI 5069 2005 MI 5070 2005 MI 5075 2005 MI 5079 2005 MI 5081 2005 MI 5082 ICSR 24005 Melkam Mean CV (%) DE 8 8 8 8 8 8 8 8 8 8 8 8 8 8 8 8 8 8 8 8 8 8 8 0 DF 81.00 81.33 80.67 80.33 81.67 80.67 82.33 79.67 80.00 81.00 81.33 80.67 80.67 79.33 80.33 82.33 80.33 81.00 81.33 79.67 78.67 80.00 80.65 2.49 DM 136.00 136.33 136.33 136.00 136.33 136.00 136.00 136.00 136.00 136.33 136.00 136.00 136.00 136.00 136.00 136.00 136.00 136.00 136.00 136.00 135.00 136.00 136.02 0.33 GY 2.84defg 2.98cdef 2.93cdef 2.17gh 3.23cde 2.15gh 3.28bcde 3.37abcd 1.97h 3.12cdef 3.65abc 3.52abcd 3.38abcd 3.31abcde 2.58efgh 4.05a 3.22cde 2.76defg 2.42fgh 4ab 3.64abc 1.91h GFR 51.56defgh 54.37cdefg 52.51defgh 39.00hi 58.94bcdef 38.93hi 61.16bcde 60.03bcdef 35.11i 56.24cdefg 66.84abc 63.7abcde 61.36bcde 58.63bcdef 46.43fghi 75.43a 57.82bcdefg 49.96efgh 44.51ghi 70.99ab 64.43abcd 33.9i 3.02 13.11 54.63 13.37 DE = Days to emergence (days), DF = Days to flowering (days), DM = Days to maturity (days), GY = Grain yield (ton/ha) and GFR = Grain filling rate (%). Any two or more means having a common letter in a column are not significantly different at 5 % level of significant in DMRT. 59 Appendix 2. Mean value of grain yield (ton/ha), phenological traitsand grain filling rate of early maturing sorghum genotypes for the data collected at Kobo during 2014 main cropping season. Entry 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Genotypes 2001 MS 7003 2001 MS 7013 2001 MS 7015 2001 MS 7037 IESV 92084-DL IESV 92168-DL IESV 92199-DL IESV 92057-DL IESV 9027-DL 2001 MS 7007 2005 MI 5060 2005 MI 5064 2005 MI 5065 2005 MI 5066 2005 MI 5069 2005 MI 5070 2005 MI 5075 2005 MI 5079 2005 MI 5081 2005 MI 5082 ICSR 24005 Melkam Mean CV (%) DE = Days to emergence (days), DF DE 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6.00 0.00 = Days to DF abcdefg DM abcdef GY abcd GFR abc 83 118 3.32 94.82 86.33abc 121.33bc 3.8a 108.89a 85abcde 121abcd 2.96cdef 82.47bcde ab a fgh 87 122.67 2.4 67.76efghi 85.33abcde 119abcdef 2.77cdef 83.25bcde 84.33abcdef 120.67abcde 2.64defg 72.97defgh abcdefg abcdef fgh 82.67 119.33 2.38 65.05efghi 77.33g 116ef 2.01gh 51.99i fg f bcdef 77.67 115.33 3.06 81.27bcde 83.33abcdefg 120.67abcde 2.39fgh 63.92efghi abcdefg cdef efgh 82.67 116.67 2.5 73.55defgh 79.33defg 117.67bcdef 3.67ab 96.58ab 80.33bcdefg 119abcdef 3.14bcde 81.43bcde abcdefg bcdef efgh 81 117.33 2.52 69.26defghi 82abcdefg 118.67abcdef 2.76cdef 75.22cdef a ab h 87.67 122 1.87 54.77ghi 84.33abcdef 121.67ab 2.77cdef 74.39defg abcdefg abcdef bcdef 83.33 119.67 3.05 83.27bcde 85.67abcd 119.67abcdef 1.91h 55.8fghi 87ab 121.67ab 1.85h 53.72hi cdefg abcdef cdef 79.67 119.33 2.7 68.43efghi 78.67efg 116.33def 3.36abc 89.12bcd 82.89 119.26 2.72 74.91 4.25 2.02 13.28 13.88 flowering (days), DM = Days to maturity (days), GY = Grain yield (ton/ha) and GFR = Grain filling rate (%). Any two or more means having a common letter in a column are not significantly different at 5 % level of significant in DMRT. 60 Appendix 3. Mean value of grain yield (ton/ha), phenological traitsand grain filling rate of early maturing sorghum genotypes for the data collected at Mieso during 2014 main cropping season. Entry 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Genotypes 2001 MS 7003 2001 MS 7013 2001 MS 7015 2001 MS 7037 IESV 92084-DL IESV 92168-DL IESV 92199-DL IESV 92057-DL IESV 9027-DL 2001 MS 7007 2005 MI 5060 2005 MI 5064 2005 MI 5065 2005 MI 5066 2005 MI 5069 2005 MI 5070 2005 MI 5075 2005 MI 5079 2005 MI 5081 2005 MI 5082 ICSR 24005 Melkam Mean CV (%) DE = Days to emergence (days), DF DE DF 5.00 74.33 5.67 74.67 5.67 72.00 5.67 71.00 5.67 74.00 5.67 74.67 5.00 72.00 5.33 76.33 6.00 72.67 6.00 71.67 5.33 75.67 5.00 76.67 5.33 72.00 5.33 72.33 5.33 71.67 5.00 69.67 5.67 75.00 6.00 73.67 5.33 78.00 5.00 72.00 5.67 71.67 5.33 72.33 5.45 73.36 8.47 4.63 = Days to flowering (days), DM GY GFR bcd bcde 125.33 2.31 45.29 cde 125.33 1.65 32.58cde bc 122.00 2.4 47.86abc 120.00 1.56de 31.87de ab 124.00 2.58 51.50ab 124.67 2.4bc 47.79abc bc 122.67 2.39 46.95abcd 126.67 2.48ab 49.1ab 123.67 1.88bcde 38.06bcde ab 121.33 2.63 52.93ab 129.00 2.58ab 48.42ab bc 131.33 2.34 42.81bcde 120.00 2.11bcde 43.92bcde ab 122.00 2.66 53.49ab 119.33 2.58ab 53.95ab 118.00 2.05bcde 42.234bcde e 124.33 1.52 30.88e 123.67 1.90bcde 38.04bcde ab 128.67 2.67 52.69ab 121.33 2.46ab 50.33ab ab 122.00 2.68 53.48ab 124.67 3.21a 61.54a 123.64 2.32 46.17 4.91 17.45 17.43 DM = Days to maturity (days), GY = Grain yield (ton/ha) and GFR = Grain filling rate (%). Any two or more means having a common letter in a column are not significantly different at 5 % level of significant in DMRT. 61 Appendix 4. Mean value of grain yield (ton/ha), phenological traitsand plant height of early maturing sorghum genotypes for the data collected at Shewa Robit during 2014 main cropping season. Entry 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Genotypes 2001 MS 7003 2001 MS 7013 2001 MS 7015 2001 MS 7037 IESV 92084-DL IESV 92168-DL IESV 92199-DL IESV 92057-DL IESV 9027-DL 2001 MS 7007 2005 MI 5060 2005 MI 5064 2005 MI 5065 2005 MI 5066 2005 MI 5069 2005 MI 5070 2005 MI 5075 2005 MI 5079 2005 MI 5081 2005 MI 5082 ICSR 24005 Melkam Mean CV (%) DE = Days to emergence (days), DF = DE DF 9.67 79.33 bcde 10.67 77.67 10cdef 77.67 def 9.67 83.67 10cdef 75.67 f 9 73.33 def 9.67 77.33 10.33bcdef 72.33 bcde 10.67 73.33 10.67bcde 73.33 cdef 10 80.00 9.33ef 75.67 def 9.67 72.00 def 9.67 79.67 11.33bc 84.00 bcdef 10.33 77.67 9.33ef 77.67 f 9 79.00 11.67b 80.33 bcd 11 76.00 a 13 76.00 9.67def 74.33 10.20 77.09 7.68 6.62 Days to flowering (days), DM = def DM GY GFR bcde bcde 132.00 4.90 92.85 bcde 131.33 4.91 92.26bcde bcde 132.00 4.92 90.7bcde 133.67 4.15efg 84.6cdef a 129.67 6.27 116.69a 129.67 6.13a 109.19ab g 132.33 3.48 63.43f 128.67 4.62cde 81.67cdef 129.00 4.52def 81.15cdef ab 132.33 5.74 97.37abcd 133.67 4.74cde 88.35bcde bcd 132.00 5.18 94.2bcde 131.33 6.06a 102.16abc bcde 131.00 4.91 96.31abcd 132.67 4.45def 91.72bcde 133.67 4.94bcde 88.22bcde cde 133.33 4.83 86.86cde 133.33 4.30defg 80.19cdef fg 131.67 3.68 73.36ef 132.33 4.68cde 83.07cdef efg 131.33 4.23 76.48def 131.00 5.50abc 96.98abcd 131.73 4.87 89.45 1.65 9.70 12.49 Days to maturity (days), GY = Grain yield (ton/ha) and GFR = Grain filling rate (%). Any two or more means having a common letter in a column are not significantly different at 5 % level of significant in DMRT. 62 Appendix 5. Means for phenological traits and plant height and of early maturing sorghum genotypes tested at four locations during 2014 main cropping season. Entry Genotypes DH DM PH SCH GFP 2001 MS 7003 79.42 127.83 177.03def 55.92ab 48.42abc 2001 MS 7013 80.00 128.58 174.62ef 45.83cdef 48.58abc cdef abcd 2001 MS 7015 78.83 127.83 179.73 51.83 49abc 2001 MS 7037 80.50 128.08 185.35abcdef 48.17bcd 47.58bc cdef abc IESV 92084-DL 79.17 127.25 179.87 54.58 48.08abc IESV 92168-DL 78.25 127.75 194.85ab 46.08cdef 49.5abc IESV 92199-DL 78.58 127.58 178.38cdef 52abcd 49abc abcdef ab IESV 92057-DL 76.42 126.83 185.08 55.58 50.42ab IESV 9027-DL 75.92 126.00 171.77f 44.08def 50.08abc ef cdef 2001 MS 7007 77.33 127.67 173.67 45.33 50.33ab 2005 MI 5060 79.92 128.83 190.98abcd 59.08a 48.92abc abcde abcd 2005 MI 5064 78.08 129.25 187.57 51.67 51.17a 2005 MI 5065 76.25 126.58 198a 48.83bcd 50.33ab 2005 MI 5066 78.08 126.58 191.01abcd 49.33bcd 48.5abc abcde f 2005 MI 5069 79.50 126.67 186.43 38.83 47.17c 2005 MI 5070 79.33 127.42 187.77abcde 54.42abc 48.08abc abc cdef 2005 MI 5075 79.33 128.83 192.02 46.08 49.5abc 2005 MI 5079 79.25 128.17 184.65abcdef 47.92bcde 48.92abc cdef cdef 2005 MI 5081 81.33 129.00 178.92 45.5 47.67bc 2005 MI 5082 78.67 127.83 182.85bcdef 49.5bcd 49.17abc ICSR 24005 76.50 126.92 186.02abcdef 39ef 50.42ab g abcd Melkam 76.33 127.00 159.5 51.75 50.67ab Mean 78.50 127.66 183.00 49.15 49.16 CV (%) 4.68 2.70 8.07 19.45 6.47 DH = Days to heading (days), DM = Days to maturity (days), PH = Plant height (Cm), SCH = Stand count at harvest (number) and GFP = Grain filling period (days). Any two or more means having a common letter in a column are not significantly different at 5% level of significant in DMRT. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 63 Appendix 6. The IPCA 1 and IPCA 2 scores for the four sites, sorted on environmental mean yield, used in the study. Location Environment Mean IPCA - 1 IPCA - 2 Errer 3.02 1.3587 Kobo 2.72 -0.7817 Mieso 2.32 0.2787 Shewa Robit 4.87 -0.8557 IPCA - 1 = Interaction Principal Component Analysis Score 1 and IPCA – Principal Component Analysis Score 2. Appendix 7. 0.159 -1.057 -0.2415 1.1395 2 = Interaction Total monthly rainfall (mm) and mean monthly temperature (°C) of the four tested locations during 2014 main cropping season. Total Rain fall (mm) Month Errer Kobo Mieso Jan. NA 1.2 Feb. NA Mar. Mean Temperature (°C) Errer Shewa Kobo Mieso S/Robit Robit Min. Max. Min. Max Min Max 0.0 2.9 NA NA 13.0 33.8 10.5 29.5 13 30.02 7.7 1.1 12.5 NA NA 15.1 28.6 15.0 30.7 16.75 30.48 NA 58.6 98.8 86.8 NA NA 15.5 28.6 16.1 31.5 17.95 32.65 Apr. NA 31.7 120.8 21.5 NA NA 16.8 28.6 17.3 31.4 19.22 34.23 May NA 0.0 64.2 76.5 NA NA 16.8 30.2 17.2 32.5 19.05 34.19 June NA 4.5 13.9 14.9 NA NA 13.7 31.8 16.7 35.0 19.23 36.23 July NA 154.2 154.2 244.9 NA NA 0.0 33.9 18.8 33.2 19.29 34.25 Aug. NA 255.8 90.1 188.1 NA NA 0.0 34.0 18.0 31.2 18.61 31.82 Sept. NA 159.7 158.7 98.2 NA NA 0.0 30.9 16.7 30.6 18 31.95 Oct. NA xx 147.5 141.9 NA NA 0.0 0.0 14.0 29.0 16.1 30.8 Nov. NA xx 7.5 25 NA NA 0.0 0.0 11.8 29.8 14.68 30.33 Dec. NA 0.0 0.0 0 NA NA 0.0 0.0 8.3 28.5 12.05 29.18 Min Max.