Systematic and random errors
advertisement

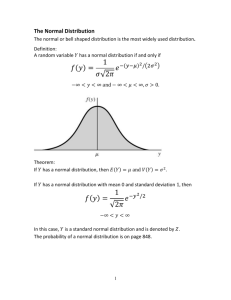
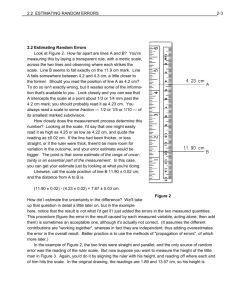
Systematic and random errors No measurement made is ever exact. The accuracy (correctness) and precision (number of significant figures) of a measurement are always limited by the degree of refinement of the apparatus used, by the skill of the observer, and by the environment where the experiment is performed. In doing experiments we are trying to establish the best values for certain quantities, or trying to validate a theory. We must also give a range of possible true values based on our limited number of measurements. We use the synonymous terms uncertainty, error, or deviation to represent the variation in measured data. Two types of errors are possible. Systematic error is the result of a miscalibration of a device, poor insulation on a calorimetry apparatus, or a measuring technique. Make the measured value larger or smaller than the "true" value. CAN be eliminated or corrected with careful design of an experiment. Example: Using a steel ruler at liquid nitrogen temperature to measure the length of a rod. The ruler will contract at low temperatures and therefore overestimate the true length. Random errors, arise from imprecision of measurements Make the measured value larger or smaller than the "true" value. These are dealt with in a statistical manner. Reporting Laboratory Data 1) Accuracy Accuracy is a measure of how close an experimental value is to a value which is accepted as correct. Uncertainty of an instrument depends on the accuracy of the instrument. Digital instruments the uncertainty is written as +/- ONE of the smallest readable digit EX : Analytical balance reads 0.0000g uncertainty of recording should be recorded as +/- 0.0001 Analog Instruments the uncertainty is written as +/- ONE HALF of the smallest readable digit EX : Thermometer reads 0.00g uncertainty of recording should be recorded as +/- 0.05 The measure of the accuracy of an experimental value is reported as Absolute Error or Percent Error. Absolute Error is just the difference between the measured and accepted values: Absolute error = Experimental Value – Accepted Value Percent error or Relative error is calculated as follows: % 𝑒𝑟𝑟𝑜𝑟 = 𝐸𝑥𝑝𝑒𝑟𝑖𝑚𝑒𝑛𝑡𝑎𝑙 𝑉𝑎𝑙𝑢𝑒−𝐴𝑐𝑐𝑒𝑝𝑡𝑒𝑑 𝑉𝑎𝑙𝑢𝑒 𝐴𝑐𝑐𝑒𝑝𝑡𝑒𝑑 𝑉𝑎𝑙𝑢𝑒 𝑋 100% Notice that the error is a positive number if the experimental value is too high, and is a negative number when the experimental value is too low. Example: Absolute error A mass which is actually 214.359206... g weighed on a triple-beam balance would have an error of 0.02 g. This measurement could be expressed as 214.36 ± 0.02 g. Sufficient figures must be reported for the quantity in order for the error to be meaningful. A mass weighed on a platform balance would be reported as 214.4 ± 0.5 g, The uncertainty is only one digit after the decimal point, the same as the precision of the instrument. On an analytical balance, the mass could be measured and reported as 214.3592 ± 0.0001 g. Example: Percent error A volume of water measured using a graduated cylinder 25.3 ± 0.5 cm3 .5/25.3*100 = 1.976%= 2.0% (one digit to the right) 2) Precision Frequently in science, an accepted or true value is not known. The accuracy of a measurement cannot be reported if an accepted value is unavailable. Since scientist don’t know how to close together their values lie. It is hoped that an experiment that can give reproducible results will also give accurate results. Certainly, if data cannot be reproduced, it cannot be reliable. Precision is a measure of how reproducible experimental measurements are. Precision is reported as Deviation or Difference of values. The Absolute Deviation or Absolute Difference of each measurement is the different of each measurement from the mean or average: Absolute Deviation = Measured Value – Mean The Average Deviation or Average Difference is the average of all of the absolute deviations. The Percent Deviation or Percent Difference is the average deviation reported as a percentage: 𝐴𝑣𝑒𝑟𝑎𝑔𝑒 𝐷𝑒𝑣𝑖𝑎𝑡𝑖𝑜𝑛 Percent Deviation = X 100% 𝑀𝑒𝑎𝑛 For example, antacid tablets were analyzed to find the amount of sodium carbonate present. The experiment was carried out three times and the following values were found: Trial 1: 1.69g Trial 2: 1.74g Trial 3: 1.68g The Mean or Average of the value is: 𝑀𝑒𝑎𝑛 = 1.69+1.74+1.68 3 = 1.70𝑔 The Absolute Deviation of each value from the mean is: Trial 1: 1.69 – 1.70 = 0.01g Trial 2: 1.74 – 1.70 = 0.04g Trial 3: 1.68 – 1.70 = 0.02g The Average Deviation is: 𝐴𝑣𝑒𝑟𝑎𝑔𝑒 𝐷𝑒𝑣𝑖𝑎𝑡𝑖𝑜𝑛 = 0.01+0.04+0.02 3 = 0.02𝑔 The Relative Deviation or Relative Difference is: 𝐴𝑣𝑒𝑟𝑎𝑔𝑒 𝐷𝑒𝑣𝑖𝑎𝑡𝑖𝑜𝑛 𝑅𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝐷𝑒𝑣𝑖𝑎𝑡𝑖𝑜𝑛 = 𝑋 100% 𝑀𝑒𝑎𝑛 𝑅𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝐷𝑒𝑣𝑖𝑎𝑡𝑖𝑜𝑛 = 0.02 𝑋 100% = 1.2% 1.70 This tells the scientist that on the average, the experiment will give values that are within 1.2% of the average (and hopefully true) value. 3) Propagation of uncertainties The overall uncertainty arising in an experiment is determined by the manner in which the data values and their associated uncertainties are processed. This is known as propagation of uncertainties through the calculation. The principle is that the overall uncertainty is the sum of the absolute uncertainties. When values are being added or subtracted, the uncertainties associated with them must be added together: e.g. initial temperature = 20.1 +/- 0.1 C final temperature = 27.9 +/- 0.1 C → temperature change = 27.9 – 20.1 +/- 0.2 C In experiments where values are being multiplied or divided, and / or when there are several measurements made – each with its own uncertainty, the absolute uncertainties must be expressed as percentage uncertainties. These can be added together, and finally converted back into absolute uncertainties. e.g. mass reading 5.456g +/- 0.001 0.001 % 𝑢𝑛𝑐𝑒𝑟𝑡𝑎𝑖𝑛𝑡𝑦 = 5.456 𝑋 100 = 0.0183% Temperature reading = 27.8C +/- 0.2C 0.2 % 𝑢𝑛𝑐𝑒𝑟𝑡𝑎𝑖𝑛𝑡𝑦 = 27.8 𝑋 100 = 0.7% → total uncertainty = sum of % uncertainties = 0.0183 + 0.7 = 0.72% So if the answer is 55.8 J, then the 𝑡𝑜𝑡𝑎𝑙 𝑢𝑛𝑐𝑒𝑟𝑡𝑎𝑖𝑛𝑡𝑦 = 0.72 100 𝑋 55.8 = 0.40 Therefore final answer = 55.8 J +/- 0.4 NOTE: A common protocol is that the final total percent uncertainty should be cited to no more than one significant figure if it is greater than or equal to 2% and to no more than two significant figures of it is less than 2%. NOTE: It is not necessary to propagate error when only calculating an average. When the final uncertainty arising from random errors is calculated, this can be compared with the experimental error as described above. If the experimental error is larger than the total uncertainty, then random error alone cannot explain the discrepancy and systematic errors must be involved. There are other protocols for combining uncertainty such as “root sum of square” calculations. These are not required in IB SL or HL Chemistry.