SIRS, Sepsis, and MODS
advertisement

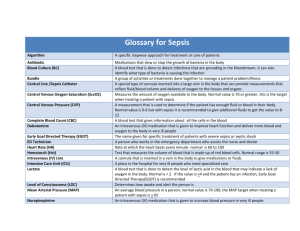
SIRS, Sepsis and MODS: Pathophysiology and Diagnosis Jonathan Sevransky, MD, MHS Associate Professor Of Medicine Emory University School of Medicine Atlanta GA jsevran@emory.edu SIRS, Sepsis and MODS: Definitions and Diagnosis The inflammatory and coagulation cascades can be triggered by infection, trauma, medications, heat exposure , toxins, and a host of other insults. Infection is the most common etiology. To standardize the nomenclature, a consensus conference in 1984 defined the relationships between the systemic inflammatory response syndrome, sepsis, and multiorgan dysfunction syndrome1. The systemic inflammatory response syndrome consists of 2 of the 4 following parameters1 T> 38 <36 Pulse >90 RR > 20 or PCO2 <32 or need for positive pressure ventilation Wbc >12 < 4 or > 10% immature “band forms” In conjunction with the evidence of an infection, a patient with SIRS would meet sepsis criteria. Of note, evidence of infection includes signs and symptoms of infections ( purulent sputum, cloudy urine) or positive gram stains or cultures: it does not require microbiologic proof. Severe sepsis is defined as sepsis with an organ failure, and septic shock as sepsis with hypotension refractory to volume resuscitation. Septic shock represents with most severe form of sepsis, and is associated with higher mortality rates than for sepsis or severe sepsis1. Increasing severity of illness is also associated with increasing incidence of organ failure1,2. While some patients with sepsis die of acute cardiac arrest, the most common cause for death in patients with sepsis is multiorgan dysfuction and failure3. Respiratory failure is the most common and severe sequalae of severe sepsis and sepsis shock, and is often accompanied by the development of the acute respiratory distress syndrome ( ARDS)2,4. Renal failure , disseminated intravascular coagulation( DIC), encephalopathy and delirium, and hypotension and shock are other important sequalea of sepsis. In order to meet the criteria of multiorgan dysfunction, more than one organ needs to fail. Of note, while individual organ dysfunction and failure is usually reversible, with an increasing number of organ failures comes an increasing risk of mortality5,6. Multiorgan dysfunction can be triggered in the absence of infection, with trauma a common precipitant1. Pancreatitis, burns, surgery are other frequent triggers. An activator event, which can include tissue injury, ischemia or reperfusion injury, or severe infection can lead to a cascade of activation of pathways such as the alarmin, pathogen associated molecular pathways ( PAMP) which can initiate microcirculatory changes as well as activation of monocytes and macrophages leading to multiorgan dysfunction7. Since the clinical picture seen in non infectious insults is often indistinguishable from that caused by sepsis, it is important to use both history and the response to therapy to help distinguish between the two. For example, it would be rare for a patient to develop an infection immediately after a sterile trauma or operation. Sepsis, SIRS, and multiorgan dysnfuction all lack defined biomarkers1,2. Thus, they are syndromes rather than diseases. Unlike other defined illness such as myocardial infarction or cerebrovascular accident, there is no clear diagnostic test, and clinicians are forced to rely on clinical suspicion to make the diagnosis. Of note, SIRS lacks specificity- other illnesses such as myocardial infarction, alcohol withdrawal can lead to SIRS. In addition, standard cultures lack sensitivity- as few as 50% of patients with severe infections leading to ICU admission will have positive cultures2. While newer diagnostic testing modes that depend upon rapid DNA amplification of bacterial products may improve sensitivity, these tests have not yet either been validated in patients, or entered routine clinical practice Pathophysiology of SIRS and Multiorgan dysfunction Both infection, inflammation and trauma can lead to activation of the host defense system , and similarly to the activation of the coagulation cascade. Multiple complementary pathways can be initiated by an activator event- leading to damage associated molecular patterns ( DAMP’s), which can lead by several pathways to multiorgan dysfunction. A recent review summarizing these pathways is listed here7. In preclinical models, this activation of multiple pathways produces a state clinically similar to sepsis, and can lead to multiorgan dysfunction and death. Blocking inflammation prior to delivering the insult can prevent the escalation of this pro-inflammatory cascade and coagulation dysregulation. Unfortunately, this paradigm of blocking inflammation prior to an insult is rarely clinically feasible and the use of either specific and non- specific anti-inflammatory therapies have not proven successful in human clinical trials8. Infection is the most common etiology of multi organ dysfunction, and activation of the host defense system is an integral part of the process that leads clinical deterioration8. While the host defense system is an important part of the development of multiorgan dysfunction, patients with impaired host defense – for example with neutropenia are both at increased risk of developing sepsis and remain at high risk for developing complications of sepsis such as sepsis- induced ARDS4. Thus, treatment designed to block the host defense system may not be useful in treatment or prevention of multiorgan dysnfuction and death2,8. Multi-organ dysfunction is defined usually by the presence of several organ dysfunctions. The precise limits of what is considered an organ dysfunction may vary: there are a number of validated organ failure and dysfunction scores, including the Sequential Organ Failure Assessment Score ( SOFA) ,and the Multiple Organ Dysfunction Score ( MODS)5,6. Most of these are centered upon laboratory values or supportive treatment requirements; it is rare that tissue is available to confirm a diagnosis5,6,9. Treatment of sepsis Sepsis is by definition triggered by an infection. Thus, the treatment of sepsis centers around 1) proper identification of the patient with sepsis 2) proper and prompt selection and delivery or antibiotics effective against the causative pathogen and 3) source control of the infection in selected patients. While these are relatively straightforward goals, the absence of a biomarker for sepsis may at times complicate the delivery of appropriate treatment for patients with sepsis. An appropriate index of suspicion for an infection is often necessary to begin treatment2. Since culture results are rarely available when treatment is initiated, the treating clinician needs to select empiric antibiotics directed at the most likely organisms. Several factors drive the choice of antibiotics10. First, the site of infection is important – the epidemiology of lung, gut, bloodstream, urinary, and CNS infections all vary. Second, the location of the patient will determine which organisms may be prevalent: the organisms causing disease in West Africa differ from those in East Asia, which may differ from those in the Eastern US. Third, whether the patient has had exposure to medical care recently will also drive the epidemiology of organisms causing sepsis. Initially, especially in the setting of severe sepsis and septic shock most clinicians will start with broad spectrum antibiotics and will narrow therapy based on culture results2. The length of antibiotic therapy is often driven by the type of organism, and the severity of illness of the patient. Some clinicians use biomarkers such as procalcitonin the help with length of therapy2,11. The importance of early appropriate antibiotic therapy in patients with severe sepsis and septic shock has been highlighted in recent studies and consensus recommendations10,12. Delivery of antibiotics ( hypotension to antibiotics infusion ) within an hour of hypotension has been shown to improve patient mortality rates in several studies, with incremental additional mortality of 6-7% for every additional hour it takes to deliver appropriate antibiotics10. Streamlining the mechanism to antibiotic delivery remains an essential part of performance improvement plans for patients with severe sepsis13. Of note, while sending cultures prior to antibiotics will be helpful for antibiotic de-escalation strategies, the clinician must guard against excessive delay caused by the need to send cultures prior. If sending cultures will prevent the timely administration of antibiotics, the treating clinician may need to give antibiotics first2. Infection source control is also an essential part of sepsis therapy2. Patients with closed space infections, or invasive supportive devices that become infection require removal of those devices and drainage of the closed space infections. When a patient is sick enough to come to the intensive care unit, it is unusual to be able to treat through an infected device or to successfully cure a closed space infection without source control. Supportive therapies are an essential adjunct to antibiotic therapy, and should commence with the identification of the patient with sepsis. Fluid resuscitation is a cornerstone of severe sepsis treatment and should commence immediately after diagnosis of severe sepsis or septic shock. A standard initial dose of crystalloid fluid , such as lactated ringers or normal saline, is 30/cc/kg, but many patients require additional fluids both initially and over the course of the first 6 hours of treatment. While colloid therapy such as albumin has theoretical advantages over crystalloid, there is no proven clinical advantage and substantial additional cost to use14. Similarly, while blood transfusion may have theoretical benefits of increasing oxygen delivery, there is no proven benefit for its use in severe sepsis or septic shock15. Some patients remain hypotensive despite volume resuscitation, and need consideration for the addition of vasopressor support. There is some evidence supporting the use of norepinephrine over dopamine for septic shock2,16. Additionally, other supportive therapies are commonly needed to treat patients with severe sepsis and septic shock such as mechanical ventilator support, renal and nutritional support. More details about these supportive therapies may be found in a recent consensus statement. Most patients with severe sepsis and septic shock will respond to therapy. For patients who do not initially respond to therapy, it is reasonable to conduct a search for undrained foci of infection, to consider whether the patient may be infected with an organism that is not responsive to the antimicrobial therapy being used. In addition, it may be reasonable to consider whether the patient may have an alternate diagnosis that is not related to an infection. While many patients will respond to therapy, some patients will have host defense systems that are not able to fight off an infection, or may have underlying diseases that will not allow effective clearance of an infection. Bibliography 1. 2. 3. 4. 5. 6. 7. 8. 9. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Critical care medicine. Jun 1992;20(6):864-874. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Critical care medicine. Feb 2013;41(2):580-637. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. The New England journal of medicine. Nov 8 2001;345(19):1368-1377. Sevransky JE, Levy MM, Marini JJ. Mechanical ventilation in sepsis-induced acute lung injury/acute respiratory distress syndrome: an evidence-based review. Critical care medicine. Nov 2004;32(11 Suppl):S548-553. Levy MM, Macias WL, Vincent JL, et al. Early changes in organ function predict eventual survival in severe sepsis. Critical care medicine. Oct 2005;33(10):2194-2201. Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA : the journal of the American Medical Association. Oct 10 2001;286(14):1754-1758. Fry DE. Sepsis, systemic inflammatory response, and multiple organ dysfunction: the mystery continues. The American surgeon. Jan 2012;78(1):1-8. Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. The Journal of clinical investigation. Aug 2003;112(4):460-467. Peres Bota D, Melot C, Lopes Ferreira F, Nguyen Ba V, Vincent JL. The Multiple Organ Dysfunction Score (MODS) versus the Sequential Organ Failure Assessment (SOFA) score in outcome prediction. Intensive care medicine. Nov 2002;28(11):1619-1624. 10. 11. 12. 13. 14. 15. 16. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Critical care medicine. Jun 2006;34(6):1589-1596. Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. The Lancet infectious diseases. Mar 2007;7(3):210-217. Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program*. Critical care medicine. Aug 2014;42(8):1749-1755. Miller RR, 3rd, Dong L, Nelson NC, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. American journal of respiratory and critical care medicine. Jul 1 2013;188(1):77-82. Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. The New England journal of medicine. May 27 2004;350(22):2247-2256. Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. The New England journal of medicine. May 1 2014;370(18):1683-1693. De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. The New England journal of medicine. Mar 4 2010;362(9):779-789.