Course File
advertisement

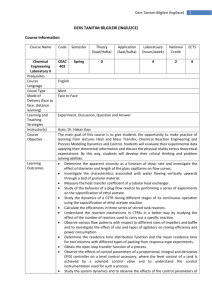
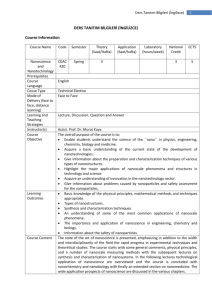
Ders Tanıtım Bilgileri (İngilizce) 1 DERS TANITIM BİLGİLERİ (İNGİLİZCE) Course Information Course Name Code Semester Theory (Saat/Hafta) Advance Analytical Chemistry Prequisites Course Language Couse Type Mode of Delivery (face to face, distance learning) Learning and Teaching Strategies Instructor(s) Course Objective CEAC 504 Spring 3 Learning Outcomes Course Content References Application (Saat/hafta) Laboratuary (hours/week) National Credit ECTS 3 5 CEAC 201 English Tech. Elective Face to Face Lecture, Discussion, Question and Answer. Assist. Prof. Dr. Murat Kaya The overall purpose of the course is to: Learn the basic principles of Analytical Chemistry with theoretical background in chemical principles that are especially pertinent to quantitative chemical analysis Develop an understanding of the range and uses of analytical methods in chemical analysis. Appreciate the statistical significance of sampling and analysis Get introduction in modern analytical instrumentation. Survey a variety of analytical techniques and methods important for all areas of chemistry, medicine, and life science. Develop skills in the scientific method of planning, developing, conducting, reviewing and reporting experiments. Develop some understanding of the professional and safety responsibilities residing in working on chemical analysis. After completing the course the student should be able to: Describe the theory of sampling, sample preparation and sample preparation techniques. Refer to the chemical theory behind the use of modern instrumental techniques for quantitative chemical analysis. Apply solid data processing and evaluation of analytical data (statistical treatment of analytical data). Develop and apply analytical methods in different field of research. Evaluate and discuss analytical chemical data from the literature. The analytical process and measurements, statistical treatment of analytical data, acid base equilibria, the solubility of precipitates, gravimetric analysis, volumetric analysis, precipitation titration, compleximetric titration, principles of oxidation reduction reactions, oxidation reduction titration, spectroscopic method of analysis, Course Book: [1] D. A. Skoog, D.M. West, Fundamentals of Analytical Chemistry, 2010 Ders Tanıtım Bilgileri (İngilizce) 2 [2] D. A. Skoog, Principles of Instrumental Analysis, 1984 Other sources: [1] A.Usanmaz, Qualitative Analytical Chemistry, 1991, METU Press [2] R. S. Drago, Physical Methods for Chemists, 1997 [3] Silverstein, Bassler, Morrill, Spectrometric Identification of Organic Compounds, 1991 Weekly Course outline Weeks 1 2, 3 4 5 6,7 8,9 10 11 12 13 15, 16, 17 Topics Introduction: Analytical Process, Concentration Solutions Statistical Treatment of Analytical data Gravimetric Analysis Acid Base Equilibria Solubility of Precipitates Neutralization as Volumetric Analysis MIDTERM I Precipitation Titration Compleximetric Titration Principles of Oxidation Reduction Reactions Oxidation-Reduction Reactions MIDTERM II Spectroscopic Method of Analysis FINAL EXAMINATION Pre-study Chapter 1 Chapter 2,3,4 Chapter 5 Chapter 7 Chapter 7,8,9 Chapter 6, 10, 12 Chapter 13 Chapter 14 Chapter 15 Chapter 17 Chapter 22-26 Assesment methods Course Activities Attendance Laboratory Application Field Activities Specific Practical Training (if any) Assignments Presentation Projects Seminars Midterms Final Exam Total Percentage of semester activities contributing grade success Percentage of final exam contributing grade success Total Number Percentage % 2 1 30, 30 40 100 60 40 100 Ders Tanıtım Bilgileri (İngilizce) 3 Course Category Core Courses Major Area Courses X Supportive Courses Media and Managment Skills Courses Transferable Skill Courses Workload and ECTS Calculation Activities Number Duration (Hours) Total Work Load Course Duration (x16) Laboratory Application Specific practical training (if any) Field Activities Study Hours Out of Class (Preliminary work, reinforcement, ect) Presentation / Seminar Preparation Projects Homework assignment Midterms ( Study duration ) Final ( Study duration ) Total Workload 16 3 48 16 5 80 2 1 20 30 40 30 198 Matrix of the Course Learning Outcomes Versus Program Outcomes Program Outcomes 1. An ability to access, analyze and evaluate the knowledge needed for the solution of advanced chemical engineering and applied chemistry problems. 2. An ability to self-renewal by following scientific and technological developments within the philosophy of lifelong learning. 3. An understanding of social, environmental, and the global impacts of the practices and innovations brought by chemistry and chemical engineering. 4. An ability to perform original research and development activities and to convert the achieved results to publications, patents and technology. 5. An ability to apply advanced mathematics, science and engineering knowledge to advanced engineering problems. 6. An ability to design and conduct scientific and technological experiments in lab- and pilot-scale, and to analyze and interpret their results. Contribution Level* 1 2 3 4 5 x x x x x x Ders Tanıtım Bilgileri (İngilizce) 7. Skills in design of a system, part of a system or a process with desired properties and to implement industry. 8. Ability to perform independent research. 9. Ability to work in a multi-disciplinary environment and to work as a part of a team. 10. An understanding of the professional and occupational responsibilities. 1: Lowest, 2: Low, 3: Average, 4: High, 5: Highest x x x x 4