(IFTA), Antibody Mediated Rejection (ABMR)
advertisement

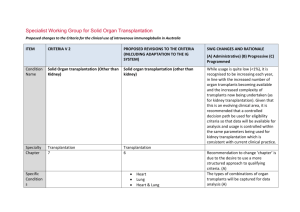
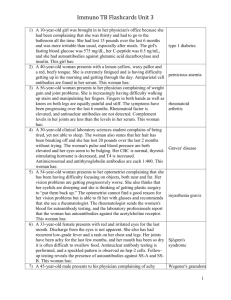
O100 Interstitial Fibrosis and Tubular Atrophy (IFTA), Antibody Mediated Rejection (ABMR) and Recurrent Disease as the Major Causes of Late Renal Allograft Loss Patrick Horgan 1, Lukas Huhn 1, David Atkinson 2, Sourabh Chand 3, Shazia Shabir 3, Hari Krishnan 3, Katy Robinson 3, Aimee Williams 3, Simon Ball 3, Desley Neil 4, Kassiani Skordilis 4, Bindu Vydianath 4, David Briggs 2, Richard Borrows 3 1 Medical School, University of Birmingham; 2 Histocompatability and Immunology Laboratory NHSBT; 3 Department of Nephrology and Kidney Transplantation, Queen Elizabeth Hospital Birmingham; 4 Department of Histopathology, Queen Elizabeth Hospital Birmingham INTRODUCTION: Recent high-profile reports suggest antibody-mediated rejection (ABMR) as the major cause of late renal allograft failure. However, these studies are limited by relatively short follow (often less than 5 years following transplantation) or the analysis of only patients who have undergone transplant biopsy, which therefore represents selection bias and is dependent on the clinical practice of the centre with regard to perceived indications for biopsy. METHODS: We retrospectively investigated 190 patients under the care of our institution, in whom allograft failure was experienced between 2008 and 2013. This time period represented an era in which testing for anti-HLA antibodies at the time of graft dysfunction and/or allograft failure became routine practice in our, and many other centres. HLA antibodies were evaluated using solid-phase assays with donor specificity for Class I and Class II antigens recorded. Where possible we used histological diagnoses in patients undergoing transplant biopsy proximate to graft failure. This included routine, specific evaluation of microvascular inflammation, C4d staining, and the use of electron microscopy. RESULTS: With a single exception (ABMR in a blood group incompatible recipient), all causes of graft failure within the first 30 days post transplantation were related to surgical complications. Between 31 days and 1 year post transplantation, the causes of graft failure were as follows: surgical n=4; ABMR n=4; recurrent disease n=3; acute cellular rejection (HLA antibody negative) n=2; Polyoma virus nephropathy n=1; medical n=2. Between 1 and 5 years, the profile of graft failure aetiologies changed markedly, with most failures attributed to ABMR (n=9) or recurrent glomerular disease (n=8). Only 2 cases of graft failure during this period were attributed to progressive IFTA. Recurrent disease was the single most common cause of failure between 5 and 10 years post transplantation (n=7). During this period the numbers of failures attributed to ABMR and IFTA were identical (n=5 for both); a further 2 graft failures were due to progressive dysfunction in the absence of an HLA antibody, and a further 3 failures due to progressive dysfunction with a non donor-specific anti HLA antibody, but not biopsied. Beyond 10 years following transplantation, 19 grafts failed due to ABMR and 19 failed due to progressive IFTA. A further 13 (not biopsied) failed in the context of progressive dyfunction with no HLA antibody detectable, and a further 10 failed due to progressive dysfunction in the presence of donor specific (n=8) or non-donor specific (n=2) HLA antibody. In all, 27 grafts failed with biopsy-proven IFTA in the absence of an identifiable concomitant inflammatory lesion. 19 of these patients were HLA antibody negative; 6 demonstrated non-donor specific HLA antibodies, and 2 demonstrated Class I HLA antibody. CONCLUSION: This study confirms the importance of ABMR and recurrent disease. However, it also demonstrates that progressive graft dysfunction and failure in the absence of either recurrent disease or detectable HLA antibody, and often with biopsy evidence of IFTA as the sole pathological lesion, is an equally important cause of late allograft failure. Modification of IFTA progression in kidney allografts warrants further attention.