empirical formula of zinc chloride lab
advertisement

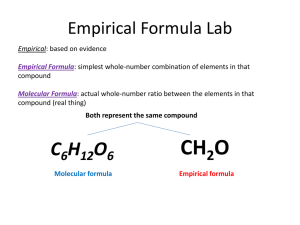
Name___________________________________________________________Per_____Date____________________ Lab Partners__________________________________________________________________Station#_____________ EMPIRICAL FORMULA OF ZINC CHLORIDE The empirical formula is the ratio formula. It is the ratio of atoms in a compound. The molecular formula is the actual number of atoms in a molecular unit. The molecular formula may be the same as the empirical formula or it may be some integer multiple of the empirical formula. The empirical formula is relatively easy to determine. If the mass of each atom is the compound is known, mass can be expressed in terms of number of moles. The mole ratio can be expressed in terms of an integer ratio which is the ratio of atoms. The ratio of atoms can be used as subscripts in the empirical formula. PROCEDURE: 1. Weigh a clean, dry 100 mL beaker and record as tare weight. Label the beaker with your period and lab station number. 2. Your instructor will instruct you as to the mass of zinc to be used and will distribute the zinc to you. Weigh the zinc to determine that you have the correct mass of zinc and record the mass of zinc that you used. Calculate the moles of zinc. 3. Use your 50 mL beaker to measure about 20 mL of 3 M hydrochloric acid (3 M HCl). (WEAR SAFTEY GOGGLES!!) Carefully pour the hydrochloric acid into the 100 mL beaker with the zinc. The acid will react with the zinc producing hydrogen gas and zinc chloride. The zinc chloride is water soluble and will remain in solution until the water is removed. Give the beaker to your instructor to dry overnight in the fume hood. 4. After the zinc chloride is dried, weigh the zinc chloride in the beaker and record. Calculate the mass of zinc chloride present and the mass of chloride (chloride is the name of combined chlorine). Calculate the moles of chlorine present. 5. Calculate the ratio of moles of chlorine to moles of zinc. Round off the mole ratio to determine the ratio of atoms of chlorine to atoms of zinc. Use the ratio of atoms as subscripts to write the empirical formula. Name___________________________________________________________Per_____Date____________________ Lab Partners__________________________________________________________________Station#_____________ EMPIRICAL FORMULA OF ZINC CHLORIDE LAB *SHOW ALL WORK/CALCULATION SET UPS IN SPACE PROVIDED* 1. Tare weight of 100 mL beaker 2. Mass of 100 mL beaker with zinc metal 3. Mass of zinc metal* 4. Moles of zinc metal* 5. Number of atoms of zinc metal* 6. Mass of 100 mL beaker with DRY zinc chloride 7. Mass of dry zinc chloride* 8. Mass of chlorine present in the dry zinc chloride* 9. Moles of chlorine present in the dry zinc chloride* 10. Number of atoms of chlorine present in the dry zinc chloride* 11. Ratio of moles of zinc to moles of chlorine* 12. Ratio of atoms of zinc to atoms of chlorine* 13. Empirical formula of zinc chloride DETERMINE WHICH OF THE FOLLOWING ARE EMPIRICAL FORMULAS (EF) OR MOLECULAR FORMULAS (MF) 14. H2S2O8 17. C6H12O6 20. C10H8 15. C4H10O 18. C2H4 21. Fe2O3 16. C9H8O4 19. TiO2 22. C4H8O2 23. Write the empirical formulas for the items you labeled as molecular formulas