Lab #2 Freezing Point Depression - NGHS
advertisement

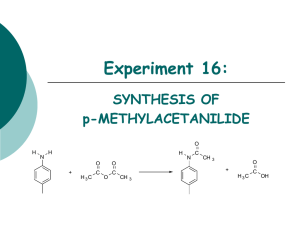
Lab #2: Introduction to Chemistry: Freezing Points Introduction: Each individual compound possesses a unique set of physical and chemical properties. Just as one human being can be distinguished from all other by certain characteristics – fingerprints, for example – it is also possible, through knowledge of its properties, to distinguish any given compound from among the many hundreds of thousands that are known. The melting point and the boiling point are easily determined properties that are very useful in identifying a substance. Consequently, these properties are almost always recorded when a compound is described in the chemical literature (textbooks, handbooks, journal articles, etc.) A pure substance will freeze (or melt) at a fixed temperature. This is the temperature at which the solid phase is in equilibrium with the liquid phase. melting Solid Liquid freezing Both melting and freezing occur at the same temperature; the terms “melting point” and “freezing point” are used to tell which process is occurring. When a small amount of a compound (solute) is dissolved in another compound (solvent) the freezing point of the resulting solution will be lower than that of the solvent. For example, solutions of sale in water may freeze at temperatures as low as -21 C (21 below the freezing point of pure water. Melting point data are of great value in determining the identity and/or purity of substances, especially in the field of organic chemistry. If a sample of a compound melts appreciably below the known melting point of the pure substance, we know the sample contains impurities which have lowered the melting point. If the melting point of an unknown compound agrees with that of a known compound, the identity can often be confirmed by mixing the unknown compound with the known and determining the melting point of that mixture. If the melting point of the mixture is the same as that of the known compound, the compounds are identical. On the other hand, a lower melting point for the mixture indicates that the two compounds are not identical. Frequently when a substance or a solution is being cooled, the temperature will fall below the true freezing point before crystals begin to form. This phenomenon is known as supercooling. Once crystallization has begun, the temperature will rise again because of the heat released by the crystallization process (heat of crystallization). In this experiment you will study the cooling and freezing behavior of pure acetic acid (glacial) and of a solution of acetic acid and benzoic acid. Acetic acid is the solvent in the solution. Materials: Benzoic acid (C6H5COOH) Glacial acetic acid (HC2H3O2) Clock with second hand Notes: 1. 2. Crushed ice thermometer stoppers with hole test tube test tube clamp ring stand Since water and other foreign substances will affect the results of this experiment adversely, use only clean, dry equipment. Read and record all temperatures to the nearest 0.1 °C. Procedure: 1. Record your hypothesis of what will happen during this experiment in your lab notebook. 2. Create a data chart based on the data you will be collecting in your lab notebook. 3. Fasten a utility clamp on to the top of a clean, dry 18x150 mm test tube. Position this clamp-tube assembly on a ring stand so that the bottom of the tube is about 20 cm above the table. 4. Obtain a one-hole stopper to fit the test tube. Insert a thermometer in the stopper and position it in the test tube so that the end of the bulb is about 1.5 cm from the bottom of the test tube. Turn the thermometer so that the temperature scale can be read easily. 5. Measure 10.0 ml of glacial acetic acid in your smallest graduated cylinder and pour the acid into the test tube. Replace the thermometer and adjust the temperature of the acetic acid to approximately 25 °C by warming or cooling the tube in a beaker of water. 6. Fill a 400 ml beaker about three-quarters full of crushed ice; add cold water until the ice is almost covered. Position the beaker of ice and water under the clamped tubethermometer assembly. 7. Read the temperature of the acetic acid and record it as the 0.0 minute time reading in your data table. Now loosen the clamp on the ring stand and lower the clamped tubethermometer assembly so that all of the acetic acid in the tube is below the surface of the ice water. Fasten the clamp to hold the tube in position. 8. Loosen the stopper on the tube and stir the acid with the thermometer, keeping the bulb of the thermometer completely immersed in the acid. Take accurate temperature readings at 30-second intervals as the acid cools. Stop stirring and center the thermometer bulb in the tube as soon as you are sure that crystals are forming in the acid (3-4 minutes). 9. Continue to take accurate temperature readings at 30-second intervals until a total time of 15 minutes has elapsed. To help maintain a consistent temperature in the ice-water bath, stir it occasionally. 10. After completing the temperature readings, remove the test tube-thermometer assembly from the ice bath, keeping the thermometer in place. Immerse the lower portion of the test tube in a beaker of warm water to melt the frozen acetic acid. Do not discard the acid; it will be used in step 9. 11. Accurately weigh 0.450 g of benzoic acid crystals. Now remove the thermometer from the test tube of acetic acid and lay it on the table, being careful not to contaminate the thermometer or lose any acid. Carefully add all of the benzoic acid to the acetic acid. Stir gently with the thermometer until all of the crystals have dissolved. Stir for an additional minute or two to ensure a uniform solution,. Adjust the temperature of the solution to approximately 25 °C. 12. Repeat steps 4-7 of this procedure to obtain the freezing point data for the glacial acetic acid-benzoic acid solution. Use a fresh beaker of ice and water for these steps. Graphing the Data: 1. Carefully plot your data in your notebook using a circle for each acetic acid point and a square for each acetic acid-benzoic acid point solution point. Draw a smooth line through the points for each set of data. Label these lines to distinguish the acetic acid cooling curve from that of the solution. 2. Identify supercooling if it is present. Questions: 1. From the plotted data, determine how many degrees the freezing point of acetic acid was depressed by the added benzoic acid. Do this by estimating to the nearest 0.1 degree the number of degrees between the most nearly horizontal portions of the curves. Mark the area on the graph to indicate where this temperature difference estimate was made. Is the temperature depression reasonable? Why or why not? 2. When the solid and liquid phases are in equilibrium, which phase contains the greater amount of energy? Explain. 3. Find the actual freezing point of acetic acid. Determine your % error and standard deviation from the correct value. 4. Using the freezing point depression formula (ΔTF = KF · b · i, where b = molality, KF is a constant, and i is the number of ions), determine what your freezing point should have been for the acetic acid/benzoic acid mixture. Determine your % error and standard deviation from the correct value. Conclusion: a. b. c. d. e. Draw your conclusions. What did your data mean and why? What did you learn? Be specific. Was your hypothesis correct? Why or why not? What went well? Why? What didn’t go so well? Why? What were your sources of error? How would you correct for them?