Mole Concept Practice: Molar Mass, Conversions, Formulas
advertisement

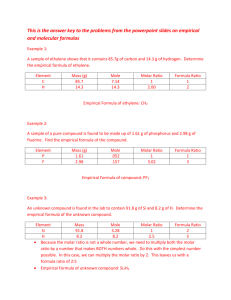
Moles! Unit 6 Practice Part 1 Molar Mass Practice- Calculate the molar mass of the following compounds: 1. CBr2 4. Mg(NO3)2 2. Carbon tetrachloride 5. SO3 3. Calcium hydroxide 6. Tetraphosphorus decoxide Mole conversions practice 1. What is the mass in grams of 2.00mol of silicon dioxide? 2. How many molecules of silicon dioxide are there in 150.21g of silicon dioxide? 3. How many silicon atoms in 180.252g of silicon dioxide? 4. How many oxygen atoms in 180.252g of silicon dioxide? 5. What is the mass in grams of 2.50mol of oxygen gas, O2? 6. How many molecules are there in 90.078g of C6H12O6? 7. How many ammonium ion, (NH4)1+, are there in 396.42g of ammonium sulfate, (NH4)2(SO4)? 8. What is the mass in grams of 3.01x1024molecules of diphosphorus pentoxide? 9. How many moles of Ca(OH)2 in 2.107x1024 formula units of Ca(OH)2? 10. How many chloride ions in 380.844g of MgCl2? Moles! Unit 6 Practice Part 2 Percent composition practice 1. Find the percentage composition of copper (I) sulfide. 2. As some salts crystallize from a water solution, they bind water molecules in their crystal structure. Sodium carbonate forms such a hydrate, in which 10 water molecules are present for every formula unit of sodium carbonate. Find the mass percentage of water in sodium carbonate decahydrate, Na2CO3•10H2O, which has a molar mass of 286.14g/mol. 3. Calculate the percent composition of sodium nitrate. a. What is the percentage by mass of sodium? b. What is the percentage by mass of nitrogen? c. What is the percentage by mass of oxygen? 4. What is the mass percentage of water in the hydrate CuSO4•5H2O? 5. Zinc chloride is 52.02% chlorine by mass. a. What mass of chlorine is contained in 80.3g of zinc chloride? b. How many moles of chlorine is this? Moles! Unit 6 Practice Part 3 Molecular and empirical formula practice 1. Qualitative analysis shows that a compound contains 32.28% sodium, 22.65% sulfur, and 44.99% oxygen. Find the empirical formula of this compound. 2. Analysis of a 10.150g sample of a compound known to contain only phosphorus and oxygen indicates a phosphorus content of 4.433g. What is the empirical formula of this compound? 3. The empirical formula of a compound of phosphorus and oxygen was found to be P2O5. Experimentation shows that the molar mass of this compound is 283.89g/mol. What is the compound’s molecular formula? 4. A sample of a compound with a formula mass of 34.00amu is found to consist of 0.44g H and 6.92g O. Find its molecular formula. 5. A compound is found to contain 63.52% iron and 36.48% sulfur. Find its empirical formula. 6. Analysis of 20.0g of a compound containing only calcium and bromine indicates that 4.00g of calcium are present. What is the empirical formula of the compound formed? 7. Find the empirical formula of a compound found to contain 26.56% potassium, 35.41% chromium, and the remainder oxygen. 8. A 60.00g sample of tetraethyl-lead, a gasoline additive, is found to contain 38.43g lead, 17.83g carbon, and 3.74g hydrogen. Find its empirical formula. 9. The empirical formula for trichloroisocyanuric acid, the active ingredient in many types of bleach, is OCNCl. The molar mass of this compound is 232.41g/mol. What is the molecular formula of trichloroisocyanuric acid? 10. Determine the molecular formula of a compound with an empirical formula of NH2 and a formula mass of 32.06amu. 11. In the laboratory, a sample of pure nickel was placed in a clean, dry, weighted crucible. The crucible was heated so that the nickel would react with the oxygen in the air. After the reaction appeared complete, the crucible was allowed to cool and the mass was determined. The crucible was reheated and allowed to cool. Its mass was then determined again to be certain that the reaction was complete. The following data were collected: Data Table 1: Calculation of Empirical Formula of Nickel Oxide Mass of crucible Mass of nickel and crucible Mass of nickel oxide and crucible 30.02g 31.07g 31.36g a. What is the mass of the nickel? b. What is the mass of the nickel oxide? c. What is the mass of oxygen? d. Based on your calculations, what is the empirical formula for the nickel oxide? e. If the molar mass of the compound is determined to be 74.692g, what is the molecular formula of the nickel oxide?