Acids, Bases, and Salts
advertisement

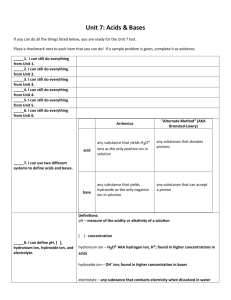
Name ________________________________________________ Topic 10 ESSENTIAL QUESTION: How do the properties and interactions of acids, bases, and salts affect our physiology and our environment? Properties of Acids and Bases Acids and Bases can be identified by observable characteristic _____________________________. Characteristic Properties of Acids: 1. Dilute acids taste ____________________. Examples: _______________________________________________________________________ 2. Aqueous solutions of acids _____________________________ electric current. a. Electrolyte: ______________________________________________________________________ b. depends on ________________________________ c. strong acid: ______________________________________________________________________ d. weak acid: _______________________________________________________________________ 3. Acids react with ______________ to form _________________ and _____________ . a. neutralization: ____________________________________________________________________ b. Neutralization is a __________________ _______________________________ reaction! 4. Acids react with certain ___________________ to produce ____________________ ____________ . a. All metals ___________ H2 on Table J will react with acids to form ___________________ ___________ 5. Acids cause acid-base _______________________ to change _______________ . Reference Table M 1 Characteristic Properties of Bases 1. Bases taste _____________________________ . 2. Bases feel _____________________________________ . 3. Bases _________________________ an _________________________ current. a. Strong base: _____________________________________________________________________ b. weak base: ______________________________________________________________________ 4. Bases react with ____________________ to form _____________________ and __________________. 5. Bases cause ___________________________________________ to change color. Reference Table M Arrhenius Theory Arrhenius acid: ___________________________________________________________________________ example: Are all substances that contain hydrogen an acid? ___________ example: ________________________________________________________________________ The Nature of the Hydrogen Ion Draw a Bohr model for hydrogen: 2 Describe a hydrogen ion using one word: __________________ Draw a hydronium ion and write its formula: How does a hydronium ion form? According to the Arrhenius theory, what causes the characteristic properties of acids? monoprotic acid: ________________________________________________________________________________ ex.) _________________________________________ diprotic acid: ___________________________________________________________________________________ ex.) _________________________________________ triprotic acid: _____________________________________________________________________________________ ex.) _________________________________________ The Nature of the Hydroxide Ion According to Arrhenius’ theory, what is responsible for the characteristic properties of bases? 3 Write the formula for ammonia: ____________ Is ammonia a base? ____________ Explain where ammonia’s hydroxide ion comes from: What are amines? _______________________________________________________________________ Are amines bases? _____________ Explain how amines form hydroxide ions: What is a hydroxyl group? __________________________________________________________________ What does a hydroxyl group look like? __________________________________________ What is the name of compounds that contain a hydroxyl group? ___________________________________ They are _______electrolytes and do NOT ionize to produce _____________________ when dissolved in water. Strength of Acids and Bases Name a strong, harmful acid: ____________________ Name a harmless acid: _______________________ Explain why some acids are stronger than others: 4 Naming Acid and Bases Binary acids: _____________________ and _______________________________________ names begin with _______________ and end with _________________________________________ Ternary acids: contain a _________________ __________ containing ___________________ If the polyatomic ion ends in –ate, then the acid ends in ____________ If the polyatomic ion ends in –ite, then the acid ends in ____________ Practice: (Use Reference Table E) HCl ____________________________________ HNO2 ____________________________________ HNO3 ____________________________________ H2SO3 ____________________________________ H2SO4 ____________________________________ H3PO4 ____________________________________ H2CO3 ____________________________________ HC2H3O2 ____________________________________ CH3COOH ____________________________________ Now look at Reference Table K. Bases are quite simple to name . . . . . . Keep the name of the positive ion, and end with “hydroxide”. Practice: NaOH _____________________________________ Ca(OH)2 _____________________________________ KOH ______________________________________ NH4OH ______________________________________ 5 Name ________________________________________________ Topic 10 Reactions Involving Acids and Bases Reactions of Acids with Metals Which metals will react with acids? Which metals will not react with acids? Which type of reaction occurs when acids react with metals? __________________________________ What gas is released when acids react with metals? _____________________________ Neutralization Reactions In a neutralization reaction, a __________________________ reacts with a __________________________ to produce a ____________________ and ____________________ . What are spectator ions? Omitting the spectator ions yields the net ionic equation: 6 Neutralization reactions are __________________ ________________________________ reactions! Remember the general formula for double replacement reactions: AB + CD AD + CB Then remember to balance the equation! Write the neutralization reaction for nitric acid and potassium hydroxide: Write the neutralization reaction for hydrochloric acid and sodium hydroxide: Write the neutralization reaction for hydrochloric acid and sodium hydroxide: 7 Name ________________________________________________ Topic 10 Acid – Base Titrations Titration: ________________________________________________________________________________________ When you know the ___________________ of either the acid or the base, and you know the ___________________________ of both solutions used for neutralization, then you can calculate the unknown _________________________ . molarity of acid x volume of acid = molarity of base x volume of base MA x VA = MB x VB Volumes do NOT need to be converted to L. Leave as mL. When you calculate the molarity of a diprotic acid (ex. H2SO4), divide your answer by 2. When you calculate the molarity of a triprotic acid (ex. H3PO4), divide your answer by _______. When you calculate the molarity of a dihydroxy base (ex. Ba(OH)2), divide your answer by ______ . 8 Practice: For the titration: KOH + HCl H2O + HOH How many milliliters of 2.0M KOH are necessary to neutralize 50.0 mL of 1.0M HCl? Write the equation AND show your work: : 10.0 mL of hydrochloric acid neutralizes 15.0 mL of a 0.40M solution of NaOH. What is the molarity of the hydrochloric acid? Write the equation AND show your work: 9 Name ________________________________________________ Topic 10 Acidity and Alkalinity of Solutions Although water is covalently bonded, it does slightly separate into ions: HOH H+ + OHNotice that this is an ____________________________ reaction. According to LeChatelier’s principle, adding H+ will ____________________ the concentration of OH-. adding OH- will ____________________ the concentration of H+. A solution is acidic when ____________________________________________________________________________ A solution is basic (alkaline) when _____________________________________________________________________ pH Scale The pH scale expresses the concentration of __________ . pH is a logarithmic. Each increment is a _________________ increase or decrease in concentration. 10 Acid-Base Indicators Indicator: _________________________________________________________________________________________ Copy Reference Table M: 11 Why is phenolphthalein used for titrations? What do you see at the exact endpoint of a titration using phenolphthalein? 12 Name ________________________________________________ Topic 10 Bronsted-Lowry Acids and Bases According to this definition, an acid is ___________________________________________________________________ According to this definition, a base is ____________________________________________________________________ How does the Bronsted-Lowry theory differ from the Arrhenius theory? Conjugate Acid-Base Pairs When a substance donates a proton, another substance must _________________________________. Examples of a conjugate pair: HNO3 is an acid. When it donates a proton, ___________ is left. That makes _______ a base because now it can accept a proton. HCl is an ____________. When it donates a proton, _______ is left. _______ counts as a base because now it can accept a proton. 13