Boyles Law prelab questions and rubric
advertisement

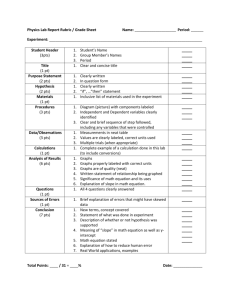
Boyles Law prelab questions 1. Describe in words the relationship between pressure and volume at a constant temperature. 2. Describe the relationship between gas pressure and volume in a mathematical equation. 3. Which of the following graphs (A,B,C,D) correctly represents the relationship between the pressure and the volume of an ideal gas that is held at constant temperature? 4. A balloon with a volume of 2.0 L is filled with a gas at 3 atmospheres. If the pressure is reduced to 0.5 atmospheres without a change in temperature, what would be the volume of the balloon? Hint: Use P i V i = P f V f where P i = initial pressure V i = initial volume P f = final pressure V f = final volume To find the final volume, solve the equation for V f 5. The volume of the lungs is measured by the volume of air inhaled or exhaled. If the volume of the lungs is 2.400 L during exhalation and the pressure is 101.70 KPa, and the pressure during inhalation is 101.01 KPa, what is the volume of the lungs during inhalation? 6. I _____________Your name____________read through Experiment 6 to gain a general understanding of how I will proceed in the lab when conducting this experiment. I understand that I will be required to complete the extension. 7. Include the data table in your lab notebook as part of the prelab (either draw or cut and paste it). However, add an additional column by dividing the last column in two. Thus, your columns should be: volume, pressure, P/V, and P*V. Experiment 6 Boyles Law Lab Rubric – 72 points Name_______________________ _____ 2 pts Lab is entered correctly in the Table of contents _____ 2 pts Lab entered into composition book according to basic lab rubric – including the objective clearly identified and stated _____ 2 pts Lab is written neatly in ink _____ 4 pts All prelab and post lab questions are answered in complete sentences and include the question. _____ 10 pts Lab is completed within one week of assigned date _____ 14 pts Prelab questions are answered correctly (Note, for question 3 include the graph that demonstrates the relationship between pressure and volume. For questions, 4 and 5 include the equation, show values substituted into the equation, circle and label the answers and report correct sig figs. Question 6, the statement is written out and truthful.) _____ 4 pts Data Table: Data table is filled in completely and units of measure are included _____ 4 pts Data Table Calculations: Calculations are shown for BOTH P/V and the P*V. The value of K should be determined using both methods. _____ 6 pts Graph: The graph is titled, axis labeled and the trend line is drawn. Graph is full page and data is graphed over the entire axis. All data points are clearly marked. Name is included on the bottom of the graph (this will be about 2 inches below the graph.) The data table is not included on the graph. _____ 2 pts Q1: Identify and record the pressure at both 5.0 ml and 10.0 ml. Explanation of behavior is provided along with numerical data to support the explanation. Calculations are shown. _____ 2 pts Q2: Identify and record the pressure at both 2.0 ml and 10.0 ml. Explanation of behavior is provided along with numerical data to support the explanation. Calculations are shown. _____ 2 pts Q 3: Identify and record the pressure at both 5.0 ml and 15.0 ml. Explanation of behavior is provided along with numerical data to support the explanation. Calculations are shown. _____ 4 pts Q 4: Clearly link data and graph to explain the relationship. Cite both sets of calculations for the value of K, and explain which the correct relationship is (direct or inverse). Justify how you know this. _____ 2 pts Q 5: Show the mathematical calculation to determine the pressure as 40.0 ml using your lab data. Original equation, substituted values and units of measure are shown. _____ 2pts Q 6: Show the mathematical calculation to determine the pressure as 25.0 ml using your lab data. Original equation, substituted values and units of measure are shown. _____ 2 pts Q 7: Identify and clearly explain two experimental factors that are held constant. _____ 2pts Q 8: Data is included in the k column of the data table. Data must include the results of both P/V and P*V calculations. _____ 2 pts Q 9: Calculate the mean and range for your k values for both the P/V and P*V calculations. Discuss which calculation should be used to determine the value of k and why. _____ 2 pts Q10: Equation that represents Boyle’s Law is shown with the substituted value of k. _____ 5 pts Extension: Graph includes a title and tabled axis. Graph is a full page and data is graphed over the entire axis.