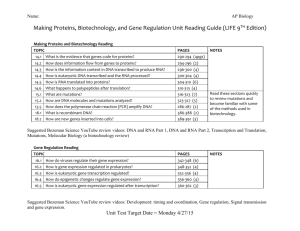
Biochemistry Exam Questions: DNA, RNA, Proteins
advertisement

BIOCHEM!!!! 4. Which of the statements regarding DNA replication is CORRECT? a) The leading strand is synthesized discontinuously from multiple primers. b) The polymerase enzyme caps the 5’ end of the nascent DNA strand. c) The polymerase adds nucleotides onto the nascent DNA strand in a hydrolysis reaction. d) Okazaki fragments on the lagging strand are composed of a mixture of RNA and DNA. e) The helicase responsible for unwinding DNA does NOT require ATP. 5. A eukaryotic DNA sequence of unknown origin contains 6 possible reading frames, 3 in the forward direction, and 3 in the reverse direction (on the complementary strand). An open reading frame is defined as the DNA sequence between two stop codons. How would you determine which open reading frame of an unknown eukaryotic DNA sequence codes for a protein? a) The open reading frame must contain a TATA box. b) The first codon following the stop codon must be an ATG. c) The longest open reading frame always codes for a protein. d) The open reading frame must contain a polyA sequence at the 3’ end. e) Impossible to tell without additional evidence. 6. Which is a DIFFERENCE in the structure of RNA compared to DNA? a) Uracil is present in DNA, not RNA. b) RNA can never be double-stranded like DNA. c) The base is attached to C1 in RNA, but not in DNA. d) The 5’ end of RNA has a phosphate group, which is not true of DNA. e) There is an hydroxyl group attached to the C2 in RNA, but not in DNA. 7. Which of the following statements concerning proteins is CORRECT? a) Multi-subunit proteins are composed of a single polypeptide. b) Alpha helical structures are stabilized by hydrogen bonding between the carbonyl oxygen and the amide hydrogen of amino acids on adjacent strands. c) Formation of a peptide bond is via a hydrolysis reaction. d) In anti-parallel beta sheets, adjacent protein chains run in the same orientation. e) The primary structure of proteins is determined by the sequence of nucleotides found in the mRNA coding for the protein. 9. You perform a manual sequencing reaction and forget to add the dideoxyribonucleoside triphosphate ddTTP. When the sequencing reactions are run on a gel, which of the following statements is TRUE? a) None of the lanes will have bands because the polymerase cannot elongate without all 4 nucleotides in the reaction mix. b) All of the lanes will have bands, but they will be incorrectly sized. c) The T lane will have no bands. d) The T lane will have mostly longer fragments. e) NONE of the above statements is TRUE. 10. Which statement concerning telomeres and telomerases is TRUE? a) Telomeres are repetitive sequences that occur throughout the chromosome. b) Telomere sequences tend to be GC-rich. c) Telomerases use a DNA template to add repetitive sequences to 3’ ends of DNA. d) It is not possible to regulate telomere length in cells. e) Telomerases are related in structure and function to reverse transcriptases. 12. Which is TRUE of RNA transcription and processing? a) RNA polymerase I transcribes protein-coding genes b) Bacterial and eukaryotic RNA polymerases are NOT homologous c) 5’ capping helps protect the ends of bacterial RNA d) RNA polymerase II is activated by phosphorylation e) The C-terminal tail of RNA polymerase II is important in proofreading 13. In an experiment that attempts to identify origins of replication in yeast, randomly selected DNA fragments are introduced into a plasmid that has a selectable marker such as the HIS gene (histidine). Yeast that have plasmids with various DNA fragments introduced are then plated on a selective medium (i.e. without histidine). What would be expected in these experiments? a) Yeast cells that have plasmids with DNA fragments without putative origins of replication should grow on media without histidine. b) The strain of yeast used for these experiments should be able to grow in the absence of histidine. c) In order to replicate, the plasmids used for these experiments do not need a DNA fragment containing an origin of replication. d) Yeast cells that have integrated the plasmid into their chromosome will be able to grow on media without histidine, regardless of whether they have a putative origin of replication or not. e) Yeast cells must be plated on medium with histidine to determine if they have plasmids with DNA fragments containing putative origins of replication. 14. The following is a diagram of the replication forks during DNA replication in prokaryotes. What is WRONG with this diagram? a) The 5’ and 3’ ends of the parental strand are labeled incorrectly. b) The 5’ and 3’ ends of the Okazaki fragments are labeled incorrectly. c) Bacterial chromosomes are not circular. d) The number of replication forks is incorrect for a circular chromosome. e) The positions of the RNA primers are incorrect. 15. The following is a gel showing the results of manual sequencing. What is the sequence of the template strand in the 5’ to 3’ direction? a) 5’ TCGCAAGCTGA 3’ b) 5’ ACGCTTGCAGT 3’ c) 5’ TCAGTTCGCGA 3’ d) 5’ TCAGCTTGCGA 3’ e) None of the above 19. Which of the following statements concerning protein synthesis is CORRECT? a) A ribosomal protein provides the enzymatic activity of peptidyltransferase. b) EF-Tu and EF-G are used in eukaryotes. c) EF-Tu is active when it is bound to GTP. d) Release factors are a special kind of tRNA that recognizes STOP codons. e) EF-Tu is involved in the recognition and binding of the START codon. 23. Which of the following sequences would be found in a primary RNA transcript, but NOT in mature mRNA? a) a poly A sequence b) a stop codon c) nucleotides upstream from the start codon d) several branch-site A residues which form the base of the excised lariat e) a start codon 24. You are interested in the pattern of expression of a specific gene called gene X in different tissues from the same organism. You isolate RNA from the tissues, run the samples of the RNA in different lanes on an agarose gel, blot and probe with a radioactive probe complementary to the exon 2 of gene X. When you perform autoradiography on the blot, you find that the lanes each have a band of a different size and some lanes have no bands. What can you conclude with certainty from the results? a) Gene X is NOT expressed in some tissues. b) There is alternative splicing of the primary transcript of gene X in different tissues. c) Within the same tissue, there is alternative splicing of the primary transcript of gene X. d) Gene X does NOT code for protein. e) Different introns of gene X are spliced out in different tissues. 25. Which statement concerning DNA replication or transcription in prokaryotes is CORRECT? a) Helicases have 6 subunits in order to stabilize single-stranded DNA during replication. b) A sigma factor directs the DNA polymerase to the origin of replication. c) RNA polymerase starts to transcribe from the start codon on the template strand. d) A primer is required for the initiation of transcription. e) DNA replication of bacterial chromosomes proceeds bidirectionally from one origin of replication. 26. Which of the following phenomena is NEVER associated with changes in chromatin structure? a) Ubiquitination near the carboxyl termini of histone proteins. b) Acetylation of leucine residues in the amino termini of histone proteins. c) Methylation of cytosine residues in CG dimers in the DNA of vertebrates. d) The subsequent recruitment of components of the general transcriptional machinery, such as TFIID. e) Recruitment of histone deacetylase by sequence-specific transcriptional repressors. 27. Which of the following statements is TRUE? a) Histone proteins are small proteins, rich in leucine and arginine residues. b) The nucleosome is an octamer comprised of two of each of the following proteins: H1, H2A, H2B and H3. c) A segment of DNA, 120 nucleotides in length, wraps around each nucleosome. d) The net result of chromatin formation is that in each mitotic chromosome, DNA is 10000 fold shorter than its extended length. e) There are 3 million base pairs of DNA in the human genome, which means that there are approximately 2 metres of DNA contained within the nucleus of a human cell. 28. Which of the following statements is FALSE? a) Transcriptomics is the study of the transcriptome, and can include experimental approaches such as microarray analysis. b) When microarrays are used to compare the transcriptome of two cells within the same individual, many genes in the two cells show equivalence in transcript abundance, and this information is used to establish which genes show statistical differences in transcript abundance. c) In microarray experiments that use red and green fluorescent dyes to label the two different RNA populations, spots that appear yellow when the microarray is scanned indicate that the gene had equivalent transcript abundance in the two different RNA populations. d) A clusterogram is a way of clustering microarray data to indicate which genes show similar patterns of transcript abundance across multiple conditions. e) In microarray experiments that use red and green fluorescent dyes to label the two different RNA populations, the scanned microarray reveals absolute levels of transcript abundance. 29. Which of the following statements is TRUE? a) Sequence-specific DNA-binding proteins always bind to the minor groove in the DNA double helix. b) The minor groove presents four different configurations of hydrogen-bond donor, hydrogen-bond acceptor, hydrogen atom and methyl group; whereas, the major groove presents two. c) Some gene regulatory proteins have DNA-binding motifs that are spaced by 3.4nm, as this places the motifs on the same side of the double helix. d) Amino acids like asparagine and arginine can not participate in the interaction between proteins and DNA because of their charge. e) Rigidity in the DNA molecule prevents conformational change when DNAbinding proteins bind to DNA. 30. Through promoter (gene regulatory sequence) deletion analysis, it was found that deletion of a region of DNA resulted in the loss of gene expression. When this region was fused to a minimal promoter and a reporter gene, and the entire construct introduced by genetic engineering back into the same organism, there was no expression of the reporter gene. These experiments: a) show that the region of DNA is unnecessary and sufficient for gene expression. b) show that the region of DNA is unnecessary and insufficient for gene expression. c) show that the region of DNA is necessary and insufficient for gene expression. d) show that the region of DNA is necessary and sufficient for gene expression. e) reveal nothing about the DNA sequence. 31. What does NOT happen in a binding site selection assay between a DNAbinding protein and DNA? a) Increasing concentrations of unlabelled competitor DNA are added to examine the specificity of the interaction. b) The process starts by allowing a protein to interact with a pool of random oligonucleotides that have been radioactively labelled. c) Shifted bands are excised from the gel and the DNA used as template in a PCR reaction. d) Following the final round of selection, the DNA is cloned into plasmids and the inserts from many plasmids are sequenced. e) The binding site is enriched in each round of selection, ultimately revealing the subset of DNA sites that the protein is most likely to bind. 33. When there is a high concentration of tryptophan, the following will occur at the E. coli trp operon: a) The RNA for trpC alone will be produced. b) The trp repressor is unable to bind to the operator site. c) RNA polymerase binds to the promoter, and transcribes genes in the trp operon. d) Enzymes made by the trp operon will break down tryptophan. e) Tryptophan functions as a co-repressing ligand that will be bound by the trp repressor. 34. A mutation in the lac operator sequence prevents the lac repressor from binding. What would you expect to see in bacteria harbouring this mutation? a) The bacteria would have difficulty growing in media containing only lactose. b) In the presence of glucose and lactose, the polycistronic message for the lac operon would accumulate to its maximum level. c) The catabolite activator protein would no longer be able to bind. d) cAMP levels would be constitutively high. e) When both glucose and lactose are absent, lac permease activity would be higher in the mutant than in normal bacteria. 35. Which of the following statements do NOT account for the differences in complexity between eukaryotic transcriptional regulation and bacterial transcriptional regulation? i. Eukaryotic DNA is packaged into chromatin. ii. Eukaryotic RNA Polymerase II cannot activate transcription on its own. iii. Eukaryotic Shine-Dalgarno sequences generate complex patterns of transcriptional regulation. iv. Eukaryotic gene regulatory proteins act at a distance. v. Eukaryotic gene regulatory proteins are not regulated by ligand binding. a) i, ii, iii b) ii, iv c) iii, v d) i, ii, iv e) ii 36. Which of the following statements is FALSE? a) Eukaryotic transcriptional activator proteins tend to be modular in structure, comprised of a DNA binding domain and an RNA polymerase domain. b) Eukaryotic transcriptional activator proteins have only weak, non-specific interactions with RNA polymerase. c) Eukaryotic transcriptional activator proteins associate with a complex of proteins known as the mediator complex. d) Eukaryotic transcriptional activator proteins working together tend to give rise to transcriptional synergy. e) Eukaryotic transcriptional activator proteins can compete for DNA binding sites with transcriptional repressor proteins. 37. What is the CORRECT order for the following steps in the Chromatin Immunoprecipitation Assay? i. Break formaldehyde linkages. ii. Cross-link proteins to DNA using formaldehyde. iii. Break DNA into small fragments. iv. Sequence DNA. v. Precipitate transcription factor-DNA complexes using antibodies raised against the transcription factor. vi. Lyse cells. a) vi, ii, v, i, iii, iv b) vi, ii, v, iii, i, iv c) ii, vi, iii, v, i, iv d) vi, ii, iii, v, i, iv e) ii, vi, v, iii, i, iv 40. What statement CORRECTLY completes the following sentence? The plant COP1 protein: a) is found in the endoplasmic reticulum throughout growth and development. b) directly activates genes that cause a plant to undergo skotomorphogenesis. c) acts in the nucleus when the plant is in the light. d) forms a heterodimer with phytochrome to activate genes involved in photomorphogenesis when the plant is in the light. e) represses genes involved in photomorphogenesis when the plant is in the dark. 41. Which of the following ARE features of X chromosome inactivation? i. XIST RNA induces heterochromatin formation. ii. Hyperacetylation of histones associated with the X chromosome. iii. DNA methylation of the X chromosome. iv. Formation of Barr bodies in mammalian males, ensuring dosage compensation. v. Seeding of euchromatin formation by the X-inactivation centre. vi. Translation of XIST RNA into a DNA methylase. a) i, ii, iv b) i, ii, iii, v, vi c) ii, iii, v d) i, iii e) ii, iii, vi 42. Which of the following statements about DNA methylation in vertebrates isTRUE? a) Specific CG dinucleotides are methylated at the cytosine position. b) Specific CC dinucleotides are methylated at the first cytosine position. c) Specific CG dinucleotides are methylated at the cysteine position. d) DNA methylation silences genes by inducing euchromatin formation. e) DNA methylation locks genes in an epigenetic state that is always stably inherited from one generation of organisms to the next generation of organisms. 43. Which of the following statements about the 3’ end of mRNA is FALSE? a) Cleavage of the 3’ UTR of the mouse CTN-RNA is important for the transit of the mRNA out of the cytoplasm and into the nucleus at times of stress. b) The 3’ UTR can be important for localisation of the mRNA to specific regions of the cell. c) Proteins that bind to the 3’ UTR can negatively regulate translation. d) A deadenylating nuclease can shorten the 3’ end of the mRNA by degrading the RNA in the 3’ to 5’ direction. e) In most instances, the translation machinery requires the 3’ end of RNA to initiate translation via interactions between poly-A binding proteins and eIF-4G. 44. A siRNA from humans was found and characterised. When the siRNA was introduced as double-stranded RNA (dsRNA) back into an undifferentiated human cell line, the cells differentiated into blood cells. A siRNA with a different sequence did not have this effect; cells remained undifferentiated when dsRNA corresponding to this second siRNA was introduced into the cell line in an independent experiment. Together, these results indicate that: a) the second siRNA is not a good control for this experiment. b) the first siRNA activates a gene that is itself an activator of blood cell differentiation. c) the first siRNA represses a gene that is itself a repressor of blood cell differentiation. d) the second siRNA competes with the first siRNA to regulate blood cell differentiation. e) the RISC is not involved in the recognition of the second siRNA to cause DNA methylation. 45. The gene cI of the bacteriophage lambda was mutated so that its gene product was no longer able to bind to DNA. What would you expect? a) The helix-loop-helix domain of the cro protein would no longer bind to its target DNA. b) The bacteriophage would not be able to sustain the lytic state. c) The bacteria would always be in the lysogenic state. d) The cI gene would always be turned off by the cro protein. e) The cro protein would not be produced. 46. Which of the following statements about the synthesis of double-stranded cDNA in vitro is TRUE? a) cDNA is made by the activity of an RNA-dependent RNA polymerase. b) The synthesis of the second strand takes place after the degradation of the original RNA template by RNAse H. c) The enzyme that is used to synthesise the first strand of the cDNA is derived from the bacterial virus, bacteriophage lambda. d) The first strand of the cDNA is primed by a polyA primer that binds to the 3’ end of the RNA template. e) The first strand of the cDNA is primed by a polyT primer that binds to the 5’ end of the RNA template. 48. Which of the following statements CORRECTLY completes the following phrase? During the process of ubiquitination (ubiquitylation), a) the carboxy terminus of ubiquitin is initially activated through a high-energy thioester linkage to a cysteine side chain of the ubiquitin activating enzyme, E1. b) E1 and E2 proteins together form the ubiquitin ligase complex that binds to a degradation signal on the target protein. c) the amino terminus of ubiquitin is initially activated through a high-energy thioester linkage to a cysteine side chain of the ubiquitin activating enzyme, E1. d) E2 and E3 proteins together form the ubiquitin activating enzyme that transfers ubiquitin to E1 ligase before E1 ligase binds to the degradation signal on the target protein. e) the carboxy terminus of ubiquitin is initially activated through a high-energy thioester linkage to a cysteine side chain of the ubiquitin activating enzyme, E3. Version 22 - 21 - continued 49. In the yeast two-hybrid assay, what is the role of the GAL4 gene? a) The GAL4 gene provides the upstream activating sequences that allow the expression of the LEU gene when the “bait” and “prey” fusion proteins have interacted. b) The GAL4 gene is included on the bait plasmid as a selectable marker, as the yeast strain that is used is gal-. c) The GAL4 gene provides the coding sequences for the GAL4 protein DNAbinding domain that comprise part of the “bait” fusion protein, and the coding sequences for the GAL4 protein transcriptional activation domain that comprise part of the “prey” fusion protein. d) The GAL4 gene encodes an enzyme that is a reporter that indicates when the “bait” and “prey” fusion proteins have interacted and activated transcription. e) The GAL4 gene is used to indicate the extent of transformation efficiency when the yeast strain has been transformed with the bait plasmid. 54. Which of the following oligonucleotides will dissociate at the lowest temperature? a) ACGTACGTACGT TGCATGCATGCA b) TGCGACGCTGAG ACGCTGCGACTC c) ACTGCTGGACCT TGACGACCTGGA d) TGCATGCATGCA ACGTACGTACGT e) AATGCTTACTGTA TTACGAATGACAT 55. Assume that the following polypeptide forms an alpha helix. How many COMPLETE turns would occur in the alpha helix of the following polypeptide? VAL-GLU-ALA-ALA-LYS-ASN-ALA-LEU-LYS-HIS-VAL-LEU-GLU-ALA-VAL-PRO-VAL-ASN a) 3 b) 2 c) 6 d) 4 e) 5 57. Ribosomal RNA is transcribed from multiple copies of a specific operon inprokaryotes. Which of the following is the CORRECT order in descending size of the transcripts from that operon (from largest to smallest): a) 16S rRNA 23S rRNA 5S rRNA tRNA b) 23S rRNA 16S rRNA mRNA 5S rRNA tRNA c) 23S rRNA 16S rRNA 5S rRNA tRNA d) 16S rRNA 23S rRNA mRNA 5S rRNA tRNA e) tRNA 16S rRNA 23S rRNA mRNA 5S rRNA 58. A 34kb linear piece of DNA was cut with the following restriction enzymes HindIII and BamH1. HindIII: 14Kb, 20Kb BamHI: 4Kb, 12Kb, 18Kb HindIII + BamH1: 4Kb, 10Kb, 8Kb, 12Kb Which of the following is the CORRECT restriction map? BamHI HindIII BamHI | | | . 10Kb 12Kb 4Kb 8Kb HindIII BamHI BamHI b) | | | . 12Kb 2Kb 8Kb 12Kb BamHI BamHI HindIII c) | | | . 4Kb 10Kb 12Kb 8Kb BamHI HindIII BamHI d) | | | . 4Kb 10Kb 8Kb 12Kb HindIII BamHI HindIII e) | | | . 4Kb 10Kb 8Kb 12Kb a) 59. Which of the following would you add to a tube containing bacterial DNA if you wanted to make many copies of a portion of the 16S rRNA gene using PCR? a) dNTPs, lysozyme, buffer with Mg2+, RNA primers, and water b) dNTPs, DNA polymerase, buffer with Mg2+, DNA primers, and water c) ddNTPs, DNA polymerase, buffer with Mg2+, DNA primers, and water d) dNTPs, DNA polymerase, buffer with Mg2+, RNA primers, and water e) dNTPs, reverse transcriptase, buffer with Mg2+, DNA primers, and water 60. What is the BEST way to determine the size of a particular transcript in a specific cell type? a) Perform a microarray analysis. b) Perform a luciferase assay. c) Perform a Northern blot. d) Perform a Gel-Mobility Shift Assay (or EMSA) e) Perform a Chromatin Immunoprecipitation assay. 67. Which of the following DO NOT contribute to the conformation or shape of proteins? a) Disulfide bonds b) Hydrophobic interactions c) Hydrogen bonds d) Phosphodiester bonds e) Ionic bonds 71. Suppose that you want to compare your bestrophin amino acid sequence with bestrophin sequences from other organisms to see how much variation there is in the amino acid sequence at the carboxy terminal end of the protein. Which of the following is the BEST way to do this? a) Do a BlastN using your amino acid sequence, copy the sequences with the highest Blast Scores and lowest Evalues, and do a ClustalW alignment with all sequences including yours. b) Do a BlastN using your amino acid sequence, copy the sequences with the highest E-values, and do a ClustalW alignment with all sequences including yours. c) Do a BlastP using your amino acid sequence, copy the sequences with the highest Blast Scores, and do a ClustalW alignment with all sequences including yours. d) Do a BlastN using your amino acid sequence, copy the sequences with the highest E-values, and do a ClustalW alignment with all sequences including yours. e) Do a BlastP using your amino acid sequence, copy the sequences with the highest Blast Scores and lowest Evalues, and do a ClustalW alignment with all sequences including yours. 74. You have digested a plasmid with EcoRI and expect to obtain fragments 5000 bp, 3000 bp, and 2000 bp in length. You want to clone the 2000 bp fragment into another vector and to be able to do this you need to obtain 1 μg of the desired 2000 bp fragment. Given that you will be using plasmid DNA at a concentration of 0.5 μg/μl, how many μl of plasmid DNA should you add to your EcoRI digest? a) 2 μl b) 5 μl c) 10 μl d) 4 μl e) 8 μl 1. What functional groups are present on this molecule? CH2-CH2-CHO | OH A) hydroxyl and carboxylic acid B) hydroxyl and aldehyde C) hydroxyl and ketone D) ether and aldehyde E) hydroxyl and ester 6. A compound has a pKa of 7.4. To 100 mL of a 1.0 M solution of this compound at pH 8.0 is added 30 mL of 1.0 M hydrochloric acid. The resulting solution is pH: A) 7.58. B) 7.4. at pH=8.0 A/HA = 4/1 or 80mmols A / 20mmols HA C) 7.22. 30mmols of HCl will convert 30mmols of A to HA D) 6.8. this gives a final ratio of 50mmols/50mmols. pH=pK E) 6.53. 10. The chirality of an amino acid results from the fact that its alpha carbon: A) is a carboxylic acid. B) is bonded to four different chemical groups. C) is symmetric. D) is in the L absolute configuration in naturally occurring proteins. E) has no net charge. 11. Of the 20 standard amino acids, only ___________ is not optically active. The reason is that its side chain ___________. A) proline; forms a covalent bond with the amino group. B) alanine; is a simple methyl group. C) glycine; is unbranched. D) alanine; contains only hydrogen. E) glycine; is a hydrogen atom. 12. Amino acids are ampholytes because they can function as either a(n): A) polar or a nonpolar molecule. B) acid or a base. C) neutral molecule or an ion. D) transparent or a light-absorbing compound. E) standard or a nonstandard monomer in proteins. 13. For amino acids with neutral R groups, at any pH below the pI of the amino acid, the population of amino acids in solution will: A) be neutral without any charge. B) show no net charge. C) have a net positive charge. D) have positive and negative charges in equal concentration. E) have a net negative charge. 21. Prosthetic groups in the class of proteins known as glycoproteins are composed of: A) lipids. B) phosphates. C) carbohydrates. D) flavin nucleotides. E) metals (e.g., molybdenum). 22. In a mixture of the five proteins listed below, which should elute second in size-exclusion (gel filtration) chromatography? A) cytochrome c, Mr = 13,000 B) immunoglobulin G, Mr = 145,000 (next to largest) C) ribonuclease A, Mr = 13,700 D) RNA polymerase, Mr = 450,000 E) serum albumin, Mr = 68,500 23. To determine the isoelectric point of a protein, a gel is first established that: A) exhibits a stable pH gradient when ampholytes become distributed in an electric field. B) contains a denaturing detergent that can distribute uniform negative charges over the protein's surface. C) relates the unknown protein to a series of protein markers with known molecular weights, Mr. D) is washed with an antibody specific to the protein of interest. E) neutralizes all ionic groups on a protein by titrating them with strong bases. 2. A hydronium ion: A) is a hydrated proton. B) is a hydrated hydrogen ion C) has the structure H3O+. D) is the usual form of one of the dissociation products of water in solution. E) All of the above are true. 3. One hundred mL of 0.1 M NaOH is added to 55 mL of 0.2 M lactic acid. (The pKa of lactic acid is 4.1.) The resulting mixture has a pH close to: A) 2. B) 3. C) 4. D) 5. E) 6. 7. A) B) C) D) E) is a carboxylic acid. is bonded to four different chemical groups. is symmetric. is in the L absolute configuration in naturally occurring proteins. has no net charge. 8. Which of the following statements about aromatic amino acids is correct? A) On a molar basis, tryptophan absorbs more ultraviolet light than tyrosine. B) The major contribution to the characteristic absorption of light at 280 nm by proteins is the phenylalanine R group. C) Histidine's ring structure results in its being categorized as aromatic or basic, depending on pH. D) The presence of a ring structure in its R group determines whether an amino acid is aromatic or not. E) All are strongly hydrophilic. 11.In a highly basic solution, pH = 13, the dominant form of glycine is: A) NH2—CH2—COOH. B) NH3+—CH2—COOH. C) NH2—CH2—COO . D) NH3+—CH2—COO . E) NH2—CH3+—COO . 12.At the isoelectric pH of a tetrapeptide: A) the amino and carboxyl termini are not charged. B) two internal amino acids of the tetrapeptide cannot have ionizable R groups. C) the total net charge is zero. D) there are four ionic charges. E) only the amino and carboxyl termini contribute charge. 19. The single most important contribution to the stability of a protein's conformation appears to be the: A) sum of free energies of formation of many weak interactions among the hundreds of amino acids in a protein. B) entropy increase from the decrease in the number of ordered water molecules forming a solvent shell around a protein. C) sum of free energies of formation of many weak interactions between a protein's polar amino acids and surrounding water. D) maximum entropy increase from ionic interactions between the ionized amino acids in a protein. E) stabilizing effect of hydrogen bonding in a protein between the carbonyl group of one peptide bond and the amino group of another, as indicated by —C¬O|||H—N—. 20.In the diagram below, the plane drawn behind the peptide bond indicates the: A) B) C) D) E) plane of rotation around the Cα—N bond. absence of rotation around the C—N bond because of its partial double bond character. region of steric hindrance determined by the large C¬O group. theoretical space between – peptide bond. region of the peptide bond that contributes to a Ramachandran plot. A) B) C) D) E) are perpendicular to the axis of the helix. occur mainly between electronegative atoms of the R groups. occur mainly between electronegative atoms of the backbone. occur only between some of the amino acids of the helix. occur only near the amino and carboxyl termini of the helix. A) B) the presence of an Arg+ C) the presence of two Lys+ D) interactions between neighboring Asp– and Arg+ residues. 21. 22. E) interactions between two adjacent hydrophobic Val residues. 24.The three-dimensional conformation of a protein may be strongly influenced by amino acid residues that are very far apart in sequence. This relationship is in contrast to secondary structure, where the amino acid residues are: A) always side by side. B) generally near each other in sequence. C) generally on different polypeptide strands. D) generally near the polypeptide chain's amino terminus or carboxyl terminus. E) restricted to only about seven of the twenty standard amino acids found in proteins. 25.Which of the following statements concerning protein domains is true? A) They consist of separate polypeptide chains (subunits). B) They are examples of structural motifs. C) They may retain their correct shape even when separated from the rest of the protein. D) They are a form of secondary structure. E) They have been found only in prokaryotic proteins. 26. Which of the following statements concerning the process of spontaneous folding of proteins is false? A) It may be an essentially random process. B) It may involve initial formation of local secondary structure. C) It may involve initial formation of a highly compact state. D) It may involve a gradually decreasing range of conformational species. E) It may be defective in some human diseases. 31.An allosteric interaction between a ligand and a protein is one in which: A) two different ligands can bind to the same binding site. B) the binding of the ligand to the protein is covalent. C) multiple molecules of the same ligand can bind to the same binding site. D) the binding of a molecule to a binding site affects the binding of an additional molecule to the same site. E) the binding of a molecule to a binding site affects the binding properties of another site on the protein. 32. Which of the following is not correct concerning cooperative binding of a ligand to a protein? A) It is usually associated with proteins with multiple subunits. B) It is usually a form of allosteric interaction. C) It results in a non-linear Hill Plot. D) It results in a sigmoidal binding curve. E) It rarely occurs in enzymes. 36.Which of the following statements is true of enzyme catalysts? A) To be effective, they must be present at the same concentration as their substrate. B) They can increase the equilibrium constant for a given reaction by a thousand-fold or more. C) They lower the activation energy for conversion of substrate to product. D) Their catalytic activity is independent of pH. E) They are generally equally active on D and L isomers of a given substrate. 37. The role of an enzyme in an enzyme-catalyzed reaction is to: A) ensure that the product is more stable than the substrate. B) make the free-energy change for the reaction more favorable. C) increase the rate at which substrate is converted into product. D) ensure that all the substrate is converted to product. E) do none of the above. 39.The Lineweaver-Burk plot is used to: A) determine the equilibrium constant for an enzymatic reaction. B) illustrate the effect of temperature on an enzymatic reaction. C) solve, graphically, for the rate of an enzymatic reaction at infinite substrate concentration. D) solve, graphically, for the ratio of products to reactants for any starting substrate concentration. E) extrapolate for the value of reaction rate at infinite enzyme concentration. 40. In a plot of l/V against 1/[S] for an enzyme-catalyzed reaction, the presence of a competitive inhibitor will alter the: A) Vmax. B) C) D) E) intercept on the l/V axis. intercept on the l/[S] axis. curvature of the plot. pK of the plot. 41. A transition-state analog: A) resembles the transition-state structure of the normal enzyme-substrate complex. B) typically reacts more rapidly with an enzyme than the normal substrate. C) is less stable when binding to an enzyme than the normal substrate. D) stabilizes the transition state for the normal enzyme-substrate complex. E) resembles the active site of general acid-base enzymes. 42. A small molecule that decreases the activity of an enzyme by binding to a site other than the catalytic site is termed a(n): A) alternative inhibitor. B) allosteric inhibitor. C) stereospecific agent. D) competitive inhibitor. E) transition-state analog. 43. How is trypsinogen converted to trypsin? A) Two inactive trypsinogen dimers pair to form an active trypsin tetramer. B) An increase in Ca2+ concentration promotes the conversion. C) D) Proteolysis of trypsinogen forms trypsin. Trypsinogen dimers bind an allosteric modulator, cAMP, causing dissociation into active trypsin monomers. E) A protein kinase-catalyzed phosphorylation converts trypsinogen to trypsin. 45.Which of the following monosaccharides is not an aldose? A) ribose B) glucose C) fructose D) glyceraldehyde E) erythrose 46. Which of following is an anomeric pair? A) D-glucose and L-glucose B) D-glucose and D-fructose C) -D-D-glucose D) -D-L-glucose E) D-glucose and L-fructose 48. A) B) C) D) E) the galactose residue is at the reducing end. C-4 of glucose is joined to C-1 of galactose by a glycosidic bond. the compound is a D-enantiomer. the glucose is in its pyranose form. 51.The compound that consists of ribose linked by an N-glycosidic bond to N-9 of adenine is: A) a purine nucleotide. B) a pyrimidine nucleotide. C) adenosine. D) AMP E) a deoxyribonucleoside. 52.In B-DNA each deoxyribose is in the C-2' endo conformation. This means that it is A) the furanose ring is completely planar B) the 2' carbon is in the same plane as the 5' carbon C) the 2' carbon is in the same plane as the furanose ring oxygen D) the 2' carbon is on the same side of the furanose ring as the 5' carbon E) the 2' carbon is on opposite side of the furanose ring from the 5' carbon 56.In the Watson-Crick model of DNA structure (B-form DNA): A) the 5' ends of both strands are at one end of the helix, and both 3' ends are at the other end of the helix. B) the bases occupy the interior of the helix. C) a purine in one strand always hydrogen bonds with a purine in the other strand. D) G–C pairs share two hydrogen bonds. E) A–T pairs share three hydrogen bonds. 58. Which of the following is a mirror repeat? A) GGATCC CCTAGG B) GTATCC CATAGG C) GAATCC CTTAGG D) AGGTCC TCCAGG E) CCTTCC GCAAGG 59. Double-stranded regions of RNA: A) do not occur. B) can be observed in the laboratory, but probably have no biological relevance. C) can form between two self-complementary regions of the same single strand of RNA. D) have the two strands arranged in parallel (unlike those of DNA, which are antiparallel). E) are less stable than double-stranded regions of DNA. 71.The fluidity of a lipid bilayer will be increased by: A) decreasing the number of unsaturated positions. B) increasing the length of the alkyl chains. C) increasing the temperature. D) decreasing the temperature. E) increasing the CL- concentration 82. Protein kinase A (PKA) is: A) competitively inhibited by cyclic AMP. B) noncompetitively inhibited by cyclic AMP. C) activated by covalent binding of cyclic AMP. D) allosterically activated by cyclic AMP. E) affected by cyclic AMP only under unusual circumstances. 83. Which of the following does not involve cyclic AMP? A) regulation of glycogen synthesis and breakdown B) regulation of glycolysis C) signaling by epinephrine D) signaling by acetylcholine E) signaling by glucagon 84.Which of the following statements concerning cyclins is not correct? A) They catalyze the phosphorylation of proteins. B) They are regulatory subunits for enzymes that catalyze the phosphorylation of proteins. C) They are activated and degraded during the cell cycle. D) They can become linked to ubiquitin. E) They contain specific amino acid sequences that target them for proteolysis. 1) The conversion between malate and oxaloacetate plays an important role in the TCA cycle and gluconeogenesis. Which of the following statements best describes malate dehydrogenase, the enzyme that catalyzes this conversion? A) In the presence of high concentrations of NADH it favors the production of malate B) Inside the cell, it catalyzes an irreversible reaction C) It is only found in the mitochondria D) In the mitochondria, the production of oxaloacetate is favored during gluconeogenesis o E) In the mitochondria the conversion of malate to oxaloacetate is a very favorable reaction (ΔG ’ <<0) 2) Which of the following CORRECTLY describes a step in the TCA cycle? A) Increased concentration of FADH will activate the succinate dehydrogenase enzyme 2 B) The reaction catalyzed by succinate thiokinase produces one mole of GTP C) Citrate synthase is positively regulated by ATP D) Only two steps produce NADH + E) Malate dehydrogenase is positively regulated by a high NADH/NAD ratio 3) In a red blood cell which of the following enzymes are required to produce NADPH and energy from glucose 6phosphate? A) glucose-6-phosphate dehydrogenase, transketolase and glucokinase B) transaldolase, glucose 6-phosphate dehydrogenase, and hexokinase C) glucokinase, transaldolase, and phosphoglucose isomerase D) glucose 6-phosphate dehydrogenase, transaldolase and phosphofructokinase E) transaldolase, transketolase and glucokinase 4) Which of the following is NOT true about the structure of the mitochondria? A) Contains regulated uncoupler proteins in the inner membrane for heat production B) The outer membrane is permeable to ions and small proteins C) In a functional mitochondria, the intermembrane space has a low proton concentration D) The inner membrane contains a series of specialized transporters E) The mitochondrial matrix contains enzymes involved in the TCA cycle and fatty acid oxidation 5) The enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is involved in the energy transfer of the glycolytic pathway. Which of the following statements does NOT accurately describe GAPDH? A) Under anaerobic conditions it requires lactate dehydrogenase to supply NADH B) Contains a free sulfhydryl necessary to bind the substrate C) Produces a high energy intermediate that is used for substrate level phosphorylation D) Utilizes inorganic phosphate to produce 1,3-bisphosphoglycerate E) The substrate glyceraldehyde 3-phosphate is oxidized to form a high energy thioester 6) The synthesis of glycogen from glucose 6-phosphate can be CORRECTLY described by which of the following? A) Glycogenin is involved in creating 1,6-glycosidic bonds B) Requires no ATP C) Glucose 1-phosphate is first attached to uridine monophosphate via UTP D) Glycogen synthase is unphosphorylated in the presence of glucagon E) Branching enzyme creates 1,4 glycosidic linkages 7) A interesting case study is brought to your attention of a patient with an abnormally high intracellular concentration of fructose 2,6-bisphosphate. If you could completely analyze the patients metabolic pathways, which of the following would you expect to find? A) Abnormally high fructose1,6-bisphosphatase activity B) Abnormally low phosphofructokinase activity C) Normal phosphofructokinase activity D) Abnormally high phosphofructokinase activity E) Normal fructose1,6-bisphosphatase activity 8) Which of the following statements most accurately describes the processes in the liver under conditions in which gluconeogenesis (GNG) is necessary? A) The consumption of alcohol would inhibit GNG because of the excess NADH its breakdown produces B) Glucose 6-phosphatase activity would be activated by high intracellular glucose C) A pyruvate transporter will be active to bypass the phosphoglycerate kinase step of glycolysis D) Excess fructose 1,6-bisphosphate will feed forward to activate pyruvate kinase E) Cytosolic oxaloacetate would be converted to malate 11) Which of the following statements INCORRECTLY describes the first oxidative decarboxylation of the TCA cycle following the condensation of acetyl coenzyme A with oxaloacetate to create citrate? A) It involves the conversion of isocitrate to alpha-ketoglutarate B) It produces a mole of CO with each turn of the TCA cycle 2 C) It is negatively regulated by ADP D) It is a rate limiting step of the TCA cycle E) It is negatively regulated by NADH 12) Given the following reactions: o What is the ΔG ’ value for the reaction: FADH2+½ O2FAD +H2O o - (ΔG ’ = -nFΔEo’, where F = faraday’s constant=23 kcal/volt mole e ) A) +46 kcal/mol B) -46 kcal/mol C) +23 kcal/mol D) -23 kcal/mol E) -11.5 kcal/mol 13) The pyruvate dehydrogenase complex is INCORRECTLY described by which of the following? A) Produces CO , NADH and acetyl Coenzyme A 2 B) Is phosphorylated in the presence of insulin C) Requires thiamine pyrophosphate to carry carbons D) Can be directly inhibited by NADH and Acetyl Coenzyme A E) Is a target for arsenic poisoning 15) Which of the following correctly describes the enzymes and reactions involved in the entry of carbons derived from galactose into the glycolytic pathway? A) The first step is the phosphorylation of galactose by glucokinase B) Galactosemia is primarily caused by a defective epimerase C) One critical metabolite involved is also involved in glycogen production D) The uridyl transferase involved uses galactose 6-phosphate as a substrate E) Most of the galactose comes from sucrose 16) In the presence of high glucagon levels, what is the status of glycogen phosphorylase? A) Is phosphorylated, active, and sensitive to glucose 6-phosphate levels B) Is unphosphorylated, active, and sensitive to glucose 6-phosphate levels C) Is phosphorylated, inactive, and sensitive to glucose 6-phosphate D) Is phosphorylated, active, and sensitive to glucose levels E) Is unphosphorylated, inactive, and sensitive to glucose levels 17) Which of the following correctly describes the electron transport chain (ETC)? A) Inhibition of complex V stops the ETC due to build up of protons in the intermembrane space B) Coenzyme Q10 accepts electrons from complex II and Complex III C) Complex III is comprised of a TCA cycle enzyme D) Complex IV uses iron and cytochrome c as electron carriers E) All four complexes can transfer electrons and pump protons 18) Following the consumption of a high carbohydrate meal what following group of enzymes would you expect to be POSITIVELY regulated? A) pyruvate dehydrogenase, hexokinase, phosphofructokinase B) fructose 1,6-bisphosphatase, phosphofructokinase, hexokinase C) phosphoenolpyruvate carboxykinase, pyruvate kinase, pyruvate carboxylase D) glycogen phosphorylase, pyruvate dehydrogenase, glucokinase E) phosphofructokinase, phosphoenolpyruvate carboxykinase, glucokinase 19) Complex V (ATP synthase) is most accurately described by which of the following? + A) Requires NAD to transfer protons across membrane B) Is comprised of two portions, one to allow proton flow and one to synthesize ATP C) Produced ATP is released directly into the cytoplasm D) Antimycin inhibits Complex V by stopping the flow of protons E) Its inhibition has no effect on mitochondrial oxygen consumption 25) Linoleic acid and linolenic acid are essential fatty acids because A) They are precursors for palmitate. B) They are local hormones. C) They are omega-6 fatty acids. D) They have cis-double bonds beyond carbon 9. E) They are omega-3 fatty acids. 26) Which of the following statements regarding ketone body synthesis is false? A) Acetoacetate and beta-hydroxybutyrate are used as fuels by other tissues. B) Ketone bodies are hydrophobic and require a carrier for transport in the blood. C) Acetoacetate spontaneously decarboxylates because it is a beta-keto acid. D) Acetone is expired through the lungs. E) An intermediate of ketone body synthesis is formed from 3 molecules of acetyl CoA. 27) The process of fatty acid synthesis involves all of the following except: A) A large multi-enzyme complex B) Acetyl CoA carboxylase C) NADPH D) Malonyl CoA E) CAT I (carnitine acyl transferase I) 28) Dr. LaPres has decided to eliminate sugar from his diet for several days. Which of the following pathways would increase in his liver under these conditions? A) beta-oxidation, ketone body synthesis, and gluconeogenesis B) glycolysis and fatty acid synthesis C) ketone body synthesis and glycolysis D) gluconeogenesis and fatty acid synthesis E) glycolysis and beta-oxidation 29) The intermediate that serves as the key branch point between phosphosphingolipid and glycolipid synthesis is A) sphingosine B) ceramide C) phosphatidate D) arachidonate E) 1,2-diacylglycerol 30) Which of the following statements is correct? A) CTP is used to activate phosphatidate in phospholipid synthesis. B) All of the carbons in cholesterol synthesis come from malonyl CoA. C) Phospholipases are used in the synthesis of phospholipids. D) HMG-CoA synthase catalyzes the committed step of cholesterol synthesis. E) Sphingosine is derived from palmitoyl CoA and glycerol phosphate. 31) The regulation of cholesterol synthesis involves which of the following: A) glucagon dephosphorylates HMG-CoA reductase B) cholesterol increases the synthesis of HMG-CoA reductase C) cholesterol increases the synthesis of the LDL receptor D) cholesterol inhibits the activity of HMG-CoA reductase E) cholesterol inhibits the degradation of HMG-CoA reductase 2) Lipoprotein particles are able to transport lipids, which are hydrophobic, through the blood because A. they have a hydrophilic surface and a hydrophobic interior. B. they contain bile salts to emulsify the lipids. C. they attach the triacylglycerols to albumin. D. they have a phospholipid bilayer containing integral membrane proteins. E. they degrade the lipids to form ketone bodies. 3) Which of the following statements about VLDLs is correct? A. VLDLs are synthesized by the intestinal mucosal cells. B. VLDLs have apo B-100 as their only apoprotein. C. VLDLs are “scavengers” of cholesterol from tissues. D. VLDLs transport dietary lipids. E. VLDLs are converted to IDLs and ultimately to LDLs. 5) Which of the following statements describing LDL endocytosis is correct? A. It is clathrin-independent. B. LDL receptors on the cell surface bind to apo B-100 on the LDL particle. C. This process delivers primarily triacylglycerols to the cell. D. LDL particle uptake by the cell activates de novo synthesis of cholesterol in the cell. E. LDL particles can only be endocytosed by the liver. 8) Regulation of metabolic pathways includes all of the following except: A. Regulation of key enzymes in a pathway. B. Control transcription and translation to regulate enzyme amounts. C. Tissue-specific enzymes and receptors. D. Reciprocal synthetic and degradative pathways utilize the same set of enzymes. E. Compartmentation of reciprocal synthetic and degradative pathways. 9) Which of the following correctly pairs a pathway with one of its regulated enzymes? A. TCA cycle: fumarase B. beta-oxidation of fatty acids: CAT II C. glycolysis: phosphoglycerate kinase D. fatty acid synthesis: pyruvate carboxylase E. urea cycle: carbamoyl phosphate synthetase I (CPS I) 10) The molecule shown below can be converted into which of the following molecules by a transamination reaction? A. lactate B. acetyl CoA C. aspartate D. alanine E. glutamate 11) Which of the following statements about protein metabolism is incorrect? A. Amino acids are required for the synthesis of other nitrogen compounds. B. The primary function of serum albumin is as an energy store of protein. C. Essential amino acids must be obtained in the diet daily. D. Nitrogen is continuously lost from the body regardless of the protein intake amount. E. Proteins of animal origin typically have the highest biological value. 12) Which of the following enzymes is the common activator of the pancreatic zymogens involved in polypeptide breakdown in the small intestine? A. Pepsin B. Carboxypeptidase A C. Elastase D. Aminopeptidase E. Trypsin 13) The pathways of protein degradation involve all of the following except: A. The lysosomal pathway primarily degrades proteins from endocytosis. B. The attachment of ubiquitin to proteins requires ATP. C. The proteasome degrades ubiquinated proteins. D. Only one molecule of ubiquitin is attached to a protein to be degraded. E. The lysosomal pathway uses a variety of proteases to degrade proteins. 14) Which of the following statements regarding aminotransferases (a.k.a. transaminases) is incorrect? A. High serum levels of alanine aminotransferase (ALT) may indicate liver damage. B. Aminotransferase reactions are irreversible. C. Aminotransferases require a co-factor derived from vitamin B6. D. The co-factor of the aminotransferase is attached to the enzyme via a Schiff base. E. Glutamate is often a product of aminotransferase reactions. 15) Which of the following groups contains enzymes all of which are capable of producing free ammonia (NH4+)? A. amino acid oxidases, glutaminase, glutamate dehydrogenase B. carbamoyl phosphate synthetase I (CPS I), glutaminase, glutamine synthetase C. glutaminase, aminotransferases, amino acid oxidases D. glutamine synthetase, carbamoyl phosphate synthetase I (CPS I), glutamate dehydrogenase E. glutamate dehydrogenase, amino acid oxidases, aminotransferases 16) Which of the following statements about glutamate dehydrogenase is correct? A. In the liver, high levels of ATP activate glutamate dehydrogenase. B. In the liver, this enzyme fixes free ammonia onto alpha-ketoglutarate to form glutamate. C. Glutamate dehydrogenase is capable of using NAD+/NADH and NADP+/NADPH. D. Glutamate dehydrogenase is a cytosolic enzyme. E. In peripheral tissues, glutamate dehydrogenase releases free ammonia from glutamate. 17) Which of the following molecules serve as non-toxic, water-soluble nitrogen carriers to the liver? A. glutamate and aspartate B. pyruvate and alanine C. glutamine and aspartate D. alanine and glutamine E. aspartate and alanine 18) Which of the following molecules is a product of the urea cycle that can be converted back to a substrate of the urea cycle? A. aspartate B. carbamoyl phosphate C. fumarate D. malate E. oxaloacetate 19) Why is phenylalanine called a “mixed amino acid”? A. Phenylalanine degradation produces products that can be used for both glucose and ketone body synthesis. B. Phenylalanine degradation produces acetyl CoA, which can be used for glucose synthesis. C. Phenylalanine can be used to make other amino acids. D. Phenylalanine degradation produces a mixture of products. 20) A tissue’s response to insulin includes all of the following except: A. Activation of the tyrosine kinase domain of the insulin receptor B. Activation of a phosphatase C. Dephosphorylation of target proteins D. Increased levels of cAMP E. Activation, as a general rule, of target proteins 21) Which of the following statements about glucagon and epinephrine is incorrect? A. The release of glucagon and epinephrine both involve a response to low blood sugar. B. Muscle is a target tissue for both glucagon and epinephrine. C. Epinephrine is derived from tyrosine. D. Both glucagon and epinephrine bind to G-protein coupled receptors. E. Epinephrine inhibits glucose uptake from the blood in muscle tissue. 22) At 5 weeks of starvation, which of the following is occurring? A. Glycogen breakdown is the major supply of blood glucose. B. The insulin/glucagon ratio is high. C. Ketone body levels in the blood are very low. D. Lactate, pyruvate, and alanine levels in the blood have decreased. E. Fatty acid levels in the blood have decreased. 23) Which of the following enzymes is phosphorylated and inactive in response to glucagon? A. HMG-CoA reductase B. glycogen phosphorylase C. pyruvate dehydrogenase D. Both A and C E. All of the above 24) Which of the following INCORRECTLY describes a patient with Type I diabetes? A. Protein degradation and lipolysis are stimulated. B. The excess ketone bodies produced lowers the blood pH. C. Glucose uptake by the tissues is stimulated. D. Insulin is always necessary for treatment. E. Gluconeogenesis is stimulated in the liver. 25) Which of the following molecules is a carrier for fatty acyl CoA molecules into the mitochondrial matrix? A. Carnitine B. Citrate C. Aspartate D. Malate E. Glutamine 26) Which of the following statements regarding the regulation of beta-oxidation of fatty acids is correct? A. Beta-hydroxyacyl CoA dehydrogenase is activated by high NADH concentrations. B. Palmitate is an inhibitor of beta-oxidation. C. Beta-ketothiolase is activated by high acetyl CoA concentrations. D. Carnitine acyl transferase I (CAT I) is inhibited by high malonyl CoA concentrations. E. Insulin stimulates the beta-oxidation of fatty acids. 27) Which of the following situations would result in an increase in ketone body synthesis? A. High insulin/glucagon ratio B. High citrate levels in the cytosol C. High levels of fructose-2,6-bisphosphate D. Low oxaloacetate levels in the mitochondria E. Low acetyl CoA levels in the mitochondria 28) Which of the following correctly lists, in order, the enzymes that carry out the “4 repeated steps” of fatty acid synthesis? A. acyl CoA dehydrogenase, enoyl CoA hydratase, beta-hydroxyacyl CoA dehydrogenase, betaketothiolase B. condensing enzyme, beta-ketoacyl reductase, beta-hydroxyacyl dehydratase, enoyl reductase C. condensing enzyme, beta-ketoacyl dehydrogenase, beta-hydroxyacyl hydratase, enoyl dehydrogenase D. enoyl reductase, beta-hydroxyacyl dehydratase, beta-ketoacyl reductase, condensing enzyme E. condensing enzyme, beta-ketoacyl reductase, beta-hydroxyacyl hydratase, enoyl reductase 29) Which of the following statements about the fatty acid synthase complex is correct? A. The fatty acid synthase complex uses ATP. B. Acetyl CoA carboxylase is part of the complex. C. Phosphopantetheine is the cofactor of the acyl carrier protein (ACP). D. All of the carbon units used to make a fatty acid get attached to the complex as acetyl CoA. E. Biotin is a cofactor of the condensing enzyme. 31) Which of the following statements regarding phosphatidate is incorrect? A. Phosphatidate can be used for both triacylglycerol (TAG) and phospholipid synthesis. B. Phosphatidate is also called 1,2-diacylglycerol phosphate. C. The third fatty acyl group is attached to the phosphate group of phosphatidate to form a triacylglycerol (TAG). D. CTP is used to activate phosphatidate to form CDP-diacylglycerol in de novo synthesis of phospholipids. E. Phosphatidate is formed by attachment of 2 fatty acyl groups to glycerol phosphate. 32) The structure of a ganglioside consists of: A. Glycerol backbone, 2 ester-linked fatty acids, a phosphate group, and an alcohol headgroup. B. Sphingosine backbone, 2 ester-linked fatty acids, a phosphate group, and one sugar. C. Sphingosine backbone, 1 amide-linked fatty acid, a phosphate group and one sugar. D. Sphingosine backbone, 1 amide-linked fatty acid, a phosphate group and many sugars. E. Sphingosine backbone, 1 amide-linked fatty acid, and many sugars. 33) Cholesterol synthesis involves all of the following except: A. Formation of HMG-CoA B. ATP C. NADH D. Isoprene unit E. HMG-CoA reductase 35) Beta-oxidation of fatty acids includes all of the following except: A. Activation of a fatty acid to a fatty acyl CoA by fatty acyl-CoA synthetase B. FAD and NAD+ C. Vitamin B12 for beta-oxidation of odd-number carbon fatty acids D. a hydratase E. formation of a keto group on carbon 2 before cleaving off an acetyl CoA 36) Which of the molecules shown below can be made directly from pyruvate? A. Molecules 1 and 4 B. Molecules 2 and 3 C. Molecules 1, 2, and 4 D. Molecules 1, 3, and 4 E. Molecules 1, 2, 3, and 4 37) A patient is diagnosed with a mutation in the effector site of her phosphofructokinase-1 (PFK-1) gene that renders it constitutively active even in the absence of fructose 2,6-bisphosphate. What is the most likely outcome caused by this type of mutation? A. glycolysis and gluconeogenesis could happen at the same time B. metabolically, the patient would be normal C. gluconeogenesis would not be activated by high glucagon D. the feed forward activation of pyruvate kinase would be inhibited E. The patient would be unable to utilize fructose as a carbon source for glycolysis 38) A patient is taking a drug that must be activated by cytochrome p450 enzymes to be fully efficacious. A mutation that inactivates which of the following enzymes would most directly affect the patient’s ability to respond to this medication? A. pyruvate carboxylase B. glucose 6-phosphate dehydrogenase C. pyruvate kinase D. malate dehydrogenase E. citrate synthase 39) Which of the following INCORRECTLY describes the pyruvate dehydrogenase (PDH) complex? A. Is unphosphorylated in the presence of high pyruvate concentration B. Is comprised of three enzymes that utilize 5 different cofactors C. During fatty acid oxidation can be used to make pyruvate from acetyl CoA D. Insulin can turn it on by activating the PDH phosphatase E. Acetyl CoA indirectly inhibits the complex by activating the PDH kinase 40) You have been hired by a biotechnology firm as a Physician-Consultant to look into a new drug they have invented. You are told that the company’s new drug will revolutionize the diet industry because it selectively activates uncoupling proteins that perform the same function as thermogenin. Which of the following statements accurately describe what you would expect about cells treated with this drug? A. The cells would be capable of making ATP at the same rate as untreated cells. B. The membrane potential in the mitochondria would be normal C. Co-treatment with oligomycin would restore normal mitochondrial function D. Mitochondrial oxygen consumption would be normal or slightly higher E. Mitochondrial NADH and FADH2 consumption would be inhibited 41) The α-ketoglutarate dehydrogenase (αKGDH) complex is a critical step in the TCA cycle. All of the following statements accurately describe the αKGDH complex EXCEPT? A. Requires lipoic acid, thiamine pyrophosphate and FAD for function B. Can be inhibited by arsenic C. It catalyzes a rate limiting but energetically favorable reaction D. It is primarily regulated by its products, NADH and succinyl CoA E. Its substrate can be transaminated to alanine 42) Which of the following would favor full activation of gluconeogenesis? A. High levels of fatty acid oxidation B. High cellular concentrations of ADP C. High insulin levels D. Low citrate levels E. High fructose 2,6-bisphosphate 60) At which oxidation level does biotin, a cofactor in pyruvate carboxylase and acetyl CoAcarboxylase, transfer one-carbon units? A. –CH3 B. –CO2 C. –CH2D. –CH=O E. =CH62) Which two enzymes are used in the catabolism of all pyrimidine nucleotides? A. pyruvate carboxylase and acetyl CoA carboxylase B. PRPP (5-phosphoribosyl-1-pyrophosphate) synthetase and PRPP amido transferase C. 5’-nucleotidase and nucleoside phosphorylase D. ribonucleotide reductase and thymidylate synthase E. adenine phosphoribosyl transferase and hypoxanthine-guanine phosphoribosyl transferase 64) Ribonucleotide reductase is required for the synthesis of which of the following nucleotides? - c 2) A person is suffering from a mutation in a key enzyme involved in carbohydrate metabolism. You run a battery of tests and determine the patient is capable of processing glucose all the way to pyruvate at normal rates under standard conditions. During times of anaerobic stress, the patient has a decreased glycolytic capacity. Which of the following mutated enzymes is matched with the metabolite most likely to be found at abnormally high levels in this patient under anaerobic conditions? Mutated enzyme Metabolite at high concentration A) Glyceraldehyde 3-P dehydrogenase pyruvate B) Lactate dehydrogenase lactate C) Glyceraldehyde 3-P dehydrogenase dihydroxyacetone phosphate D) Lactate dehydrogenase glyceraldehyde 3-phosphate E) Phosphofructokinase pyruvate 4) Wernicke-Korsakoff syndrome is characterized by psychosis and encephalopathy and is caused by severe thiamine deficiency. Which of the following enzymes would not be affected in a patient suffering from Wernicke-Korsakoff syndrome? A) Pyruvate dehydrogenase B) Transketolase C) α-ketoglutarate dehydrogenase D) Isocitrate dehydrogenase 5) Vitamin deficiencies may affect many metabolic pathways due to the requirement of cofactors in enzymatic reactions. The Pentose Phosphate Pathway (PPP) can be run in one of four modes, depending on the specific purpose or product(s) needed (listed below). Which of these four modes of the PPP would NOT be affected by thiamine deficiency? Mode #1: NADPH + Ribose-5-P Mode #2: NADPH only Mode #3: NADPH + energy Mode #4: Ribose 5-P only A) Mode 1 B) Mode 2 C) Mode 3 D) Mode 4 E) All modes 7) The local control of glycogen phosphorylase involves which of the following? A) Phosphorylation B) Glucose C) Biological amplification D) Epinephrine E) AMP-induced activation 8) Following a 12-hour fast, all of the following are true regarding glycogen release, EXCEPT: A) Glycogen phosphorylase will be unphosphorylated B) Glycogen synthase will be phosphorylated C) Glucagon levels will be high D) Adenylate cyclase will be active E) cAMP levels will be elevated 10) You are trying to create an artificial mitochondria with a new category II prosthetic group to replace cytochrome c. You have named this new prosthetic group substance X. Given the standard redox potentials of the two half reaction: Substance X+ + 2H+ + 2e- Substance X Eo’ = 0.25 ½O2 + 2H+ + 2e- H2O Eo’ = 0.75 What is the standard free energy change of the following reaction? Substance X + ½O2 Substance X+ + H2O (Given ΔGo’ = - n FΔEo’ and F = 23 kcal/volt mol e-) A) - 46 kcal/mol B) – 23 kcal/mol C) + 46 kcal/mol D) + 23 kcal/mol E) -11.5 kcal/mol 11) Which of the following correctly describes a functioning mitochondrion? A) Will use oxygen at a constant rate regardless of ADP levels. B) Will have the same P/O ratio for NADH and FADH2 C) When thermogenin (aka UCP1) is activated, ATP generation will decrease D) In the presence of oligomycin, the membrane potential will drop E) In the presence of 2,4 dinitrophenol oxygen consumption will decrease 15) A patient is undergoing a “cleansing diet” and has not eaten in about 36 hours. He has become forgetful and seems “out of it”. He is quickly diagnosed as hypoglycemic and you conclude that the patient’s gluconeogenic pathway is defective. Upon further investigation, you determine the patient has high circulating levels of alanine, pyruvate, and lactate. You also realize there is little if any oxaloacetate being produced and biotin supplements seem to alleviate most of the symptoms. Which of the following enzymes is most likely defective in the patient? A) Pyruvate carboxylase B) Phosphoenolpyruvate carboxykinase C) Malate dehydrogenase D) Fructose 1,6-bisphosphatase E) Glucose 6-phosphatase 16) Which of the following correctly characterizes the pyruvate dehydrogenase complex? A) It uses biotin as a cofactor B) It is positively regulated by phosphorylation C) It is negatively regulated by AMP D) Its product can be converted directly into glucose E) It is critical for deciding the fate of pyruvate within the cell 17) Which of the following correctly describes the malate-aspartate shuttle following a carbohydrate laden meal? A) Malate will be transported into the cytoplasm B) The production of oxaloacetate will be favored in the matrix of the mitochondria C) Glutamate-oxaloacetate transaminase will be inactive D) Aspartate will be transported into the matrix of the mitochondria E) The reducing equivalents of NADH will be shuttled to the cytoplasm 18) Regulating the fate of pyruvate is essential for metabolic regulation. Which of the following correctly describes the possible fates of pyruvate within a cell? A) Is converted to lactate in the mitochondria B) Can be formed from acetyl-CoA via the pyruvate dehydrogenase complex running in reverse C) Can be converted to malate via malate dehydrogenase D) Can be converted to phosphoenolpyruvate via pyruvate kinase E) In the presence of oxygen, donates enough carbon for one complete turn of the TCA cycle 19) What is the correct enzyme name for the reaction drawn below? A) acetoacetate dehydrogenase B) β-hydroxybutyrate reductase C) β-hydroxybutyrate dehydrogenase D) acetoacetate reductase E) β-hydroxybutyrate synthase 20) Which of the following statements regarding the ketone bodies acetoacetate and β-hydroxybutyrate is INCORRECT? A) The position of the keto group on the β-carbon of acetoacetate allows it to spontaneously decarboxylate to form acetone. B) Tissues can utilize acetoacetate and β-hydroxybutyrate as fuel sources. C) The reduction of acetoacetate to β-hydroxybutyrate allows more ketone bodies to be available as a fuel source. D) The reduction of acetoacetate to β-hydroxybutyrate in the liver involves the corresponding oxidation of NADPH + H+ to NADP+. E) HMG-CoA lyase produces acetoacetate, the first ketone body formed. The following 2 questions refer to the pathway drawn above. The letters in brackets [ ] represent additional substrates and products for the reactions indicated. 21) Which of the following statements regarding the above pathway is CORRECT? A) Biotin provides the “long arm” of the complex that carries out these reactions. B) Reaction 3 is a hydration reaction. C) The committed step of this pathway produces the three carbon unit of molecule B. D) The enzymes that carry out reactions 2 and 4 are dehydrogenases. E) Reaction 2 requires NAD+ (molecule [H]) and Reaction 4 requires FAD (molecule [K]). 22) In a liver cell, molecule F (after it is released) could be immediately used by that same liver cell for which of the following pathways: A) Ketone body synthesis only B) Triacylglycerol synthesis only C) Phospholipid synthesis only D) Triacylglycerol synthesis or phospholipid synthesis E) Ketone body synthesis or triacylglycerol synthesis or phospholipid synthesis 23) What are the products of beta-oxidation of a C14 fatty acid? A) 1 propionyl CoA, 6 acetyl CoA, 6 FADH2, and 6 (NADH + H+) B) 8 acetyl CoA, 7 FADH2, 7 (NADH + H+) C) 6 acetyl CoA, 5 FADH2, 5 (NADH + H+) D) 7 acetyl CoA, 7 FADH2, 7 (NADH + H+) E) 7 acetyl CoA, 6 FADH2, 6 (NADH + H+) 24) What is the correct sequence of enzymes for the four repeated steps of β- oxidation of fatty acids? A) carnitine acyl transferase I (CAT I), carnitine acyl transferase II (CAT II), β-hydroxyacyl-CoA, β-ketothiolase B) acyl CoA dehydrogenase, enoyl CoA dehydratase, β-hydroxyacyl CoA dehydrogenase, β-ketothiolase C) β-ketoacyl ACP synthase, β-ketoacyl ACP reductase, β-hydroxyacyl ACP dehydratase, enoyl ACP reductase D) acyl CoA dehydrogenase, enoyl CoA hydratase, β-hydroxyacyl CoA dehydrogenase, β-ketothiolase E) acyl CoA reductase, enoyl CoA hydratase, β-hydroxyacyl CoA reductase, β-ketothiolase 25) In a test tube, malonyl CoA which was 14C labeled (a radioactive isotope of carbon) at carbon #3 and unlabeled acetyl CoA were mixed together with the fatty acid synthase complex. Which of the carbons of the final product, palmitate, will be 14C labeled? A) All of the carbons will be labeled B) None of the carbons will be labeled C) Only the methyl carbon (carbon #16) will be labeled D) All of the even numbered carbons will be labeled, except for carbon # 16 E) Carbons #1 through #14 will all be labeled 26) Which of the following pathways is correctly paired with one of its regulated enzymes? A) fatty acid synthesis: acyl-CoA synthetase B) β-oxidation: carnitine acyl transferase I (CAT I) C) cholesterol synthesis: HMG-CoA lyase D) bile salt synthesis: β-ketothiolase E) ketone body synthesis: acetyl CoA carboxylase 27) The lipid soluble vitamins A) are all derived from cholesterol. B) are all capable of being synthesized in the body, though not in sufficient quantities. C) obtained in the diet are packaged into chylomicrons for delivery to tissues. D) are readily excreted from the body. E) include all of the B vitamins. 28) Which of the following transporters is correctly matched with what it transports? A) Carnitine: fatty acids B) LDLs: dietary triacylglycerols (TAGs) C) Citrate: malonyl CoA D) Albumin: ketone bodies E) Glycerol-phosphate: protons (H+) 29) The regulation of cholesterol synthesis involves all of the following EXCEPT: A) glucagon activates HMG-CoA reductase B) cholesterol inhibits the synthesis of HMG-CoA reductase C) cholesterol increases the degradation of HMG-CoA reductase D) the dephosphorylated form of HMG-CoA reductase is active E) cholesterol inhibits the synthesis of the LDL receptor 31) Which of the following are all POSITIVE regulators of fatty acid synthesis? A) ATP, citrate, glucagon B) Insulin, citrate, ATP C) Palmitoyl CoA, AMP, insulin D) Glucagon, epinephrine, citrate E) Palmitoyl CoA, AMP, glucagon 32) Which of the above molecules is the key intermediate (“branch point”) in the pathways of phosphosphingolipid and glycolipid synthesis? A) molecule 1 B) molecule 2 C) molecule 3 D) molecule 4 E) molecule 5 GENETICS!!!! 87. After double fertilization occurs in an angiosperm: a. the embryo is haploid b. the endosperm is triploid c. the embryo is triploid d. the endosperm is diploid e. the endosperm is haploid 27. A simple transposon: a. is a DNA segment that codes for an enzyme for its own insertion into other DNA sequences b. is a DNA segment that codes for several genes, including one for its own insertion c. is found only in chloroplasts d. is the kind that never moves around in the genome e. codes for the lactose operon 28. A bacterial plasmid is: a. the same as a transposable element b. as large as the bacterial chromosome c. an extrachromosomal piece of DNA d. never inserted into the bacterial chromosome e. a control element for crossing over 29. Restriction enzymes are: a. proteins that cut double-stranded DNA b. proteins that connect different DNA molecules c. cloning vectors d. proteins that confer antibiotic resistance e. conjugation factors 301. Recombinant DNA is defined as a DNA that is replicated in host bacteria. b DNA that has been rearranged by recombination. c DNA of plasmid vectors. d DNA cut with restriction enzymes and transformed. e DNA from two different organisms that is artificially combined. 302. A pure-breeding collie (long-haired dog) is bred to a pure-breeding pointer (short-haired dog). The puppies have a variety of intermediate hair lengths; some are much shaggier than others. What kind of genetic effect is this? a b c d e epistasis pleiotropy pseudoautosomal inheritance variable expressivity incomplete penetrance 303. Pedigree analysis of a large human family reveals that two rare traits, green eyes and bent toes, are linked. Which statement below would you expect to be true about this pedigree? a Green eyes and bent toes are linked because of lots of crossing over in the family. b Most people in the family will be both green-eyed and bent-toed. c The pedigree will have many recombinant individuals. d Most people in the family who have green eyes will also have bent toes. e Bent toes and green eyes will be sex-linked. 304. Which of the following statements about bacterial conjugation is incorrect? a F+ cells act as genetic donors. b Genes on the F factor encode proteins for cell-cell contact and the formation of sex pili. c F+ cells convert F- cells into F+ cells. d F' cells have especially high rates of recombination e The F factor is transferred as a single strand. 305. A circular DNA molecule has n target sites for restriction enzyme EcoRI. How many fragments will be produced after complete digestion? a n –1 b n c n+1 d 2n – 1 e Depends on the number of BamHI sites 307. Transduction changes a bacterium when: a there is a double crossover between the DNA injected by a special transducing phage and the bacterial chromosome. b a phage integrates into the bacterial chromosome. c a phage integrates into the bacterial chromosome and then excises itself. d a phage cannot switch to the lysogenic lifestyle. e fragments of DNA from a dead cell are cannibalized by another cell. 309. Organellar genomes differ from nuclear genomes in all but which of the following ways? a They have some unique tRNA genes. b There are many copies inside each cell. c They have all the genes needed to survive as a free-living bacterium. d They tend to mutate at a faster rate. e They have a unique genetic code. 310. Which of the following is TRUE about recombination in bacteria? a It occurs between paired chromosomes. b It is always reciprocal. c It occurs during Meiosis I. d It occurs each time an Hfr cell transfers DNA to a recipient cell. e It occurs only after a cell has become partially diploid. 312. DNA is transformed: a by passive entry into any bacterial cell. b by active import into a few bacterial cells. c into all bacterial cells that have a "competence factor". d only into competent bacteria that are F+. e by means of bacteriophages 313. In general, DNA methylation in eukaryotes is a greatest in actively transcribed genes. b a way of distinguishing "native" from "foreign" DNA. c unimportant. d performing a different function than it does in prokaryotes. e limited to maternally inherited chromosomes. 314. Which of the following techniques or reagents is NOT a necessary part of dideoxy sequencing? a a mixture of deoxy- and dideoxy-nucleotides b DNA polymerase c hybridization d gel electrophoresis e detection of labeled DNA 315. In FISH analysis, a cloned probe of a single-copy gene hybridizes to cells in mitotic metaphase. How many fluorescent spots will be seen in one cell? a 1 b 2 c 3 d 4 e Too many to count 316. Which of the following statements about restriction endonucleases is NOT true? a each r.e. enzyme has a single characteristic cutting site b they usually cut only at palindromic sites c they usually make staggered cuts that leave "sticky" ends d they can be used to analyze overlap between clones e they were created by modern genetic engineering techniques 401. A kind of regulatory element that acts far from its target gene by making a diffusable protein product is a(n) a enhancer b transcription factor gene c promoter d operator e operon 405. A promoter mutation (P-) results in a no transcription b no translation c inducible transcription d constitutive transcription e transcription but no translation 406. Suppose that an exceptional girl is born who has three copies of chromosome 22, and she survives to early adulthood. Which of the following characteristics would you NOT expect to find in this woman? a Lower than average fertility b Health problems involving multiple organ systems c Uneven pairing of some chromosomes during meiosis d Two Barr bodies per cell e Production of some unbalanced gametes (eggs) 407. Which of the following statements about excision repair is incorrect? a Excision repair is always initiated by specific enzymes that recognize damaged DNA. b Excision repair removes a region of DNA that extends beyond the damaged bases on either side. c The gap resulting from an excision must be filled in by special repair enzymes (e.g. endonucleases). d Excision repairs all require an undamaged template strand. e Excision repair usually results in a perfectly restored DNA helix. 408. Lambda phage (l) will follow the ______ lifestyle if _____. a lysis; the lysogeny operon is always expressed b lysogeny; all the promoter sites are defective c lysis; all of its operons are repressed d lysis; the 1st gene of the lysis operon makes enough repressor to block e lysogeny; the lysis operon is making lots of repressor protein lysogeny 409. Which of the following factors will have NO effect on whether a population is in Hardy Weinberg equilibrium? a lots of mutations b c d e lots of migration assortative mating many polymorphic loci small population size 410. In bacteria, enzymes used for anabolism (building up of molecules) are usually made by ______ genes or operons, while enzymes used for catabolism (breakdown of molecules) are usually made by ______ genes. a positively controlled; negatively controlled b negatively controlled; positively controlled c structural; control d repressible; inducible e inducible; repressible 411. Eukaryotes have fewer different kinds of DNA repair systems than prokaryotes because a they have larger and more complex promoters. b they mutate less often because their cells are more protected from the environment. c they are not totally dependent on the survival of any one cell. d their repair systems are more sophisticated, and so require fewer backups. e unlike prokaryotes, they can use some of the same DNA polymerases and ligases that are involved in normal replication. 412. Eukaryotic transposons (DNA elements) are different from eukaryotic retrotransposons (RNA elements) in what way? a They are structurally more like viruses than are retrotransposons. b They are always smaller. c They can occur many times in the host genome. d Their movement is dependent on the presence of specific enzyme. e Some of them can move by excising and reinserting the original element. 413. Which statement about microarray experiments is FALSE? a They are based on DNA:DNA hybridization b They are based on competitive hybrization using two different pools of c They used fluorescent labeling d They can be used to scan the expression of many genes simultaneously e They can be useful in screening for genetic diseases nucleic acids 414. The Luria-Delbrueck fluctuation test showed that a mutations arise in response to environmental induction. b mutations occur more frequently in some strains of E. coli. c phage T1 can cause E. coli DNA to mutate more often than normal. d more resistant E. coli cells arise in large cultures than in small cultures. e E. coli cells can mutate randomly at any time. 416. Which of the following statements about the phenotypic effects of mutations is TRUE? a Genes can have multiple mutated alleles that are all equally functional. b A mutation in a mammal's skin cell is never harmful because it is not passed on to offspring. c In eukaryotes, silent mutations usually have severe deleterious effects. d If a mutation occurs in a somatic cell, the mutant phenotype will be passed on to the offspring of that organism. e Null alleles are generally dominant. 1. A cross between two true breeding lines one with dark blue flowers and one with bright white flowers produces F1 offspring that are light blue. When the F1 progeny are selfed a 1:2:1 ratio of dark blue to light blue to white flowers is observed. What genetic phenomenon is consistent with these results? a. epistasis b. incomplete dominance c. codominance d. inbreeding depression e. random mating 2. Mutations which occur in body cells which do not go on to form gametes can be classified as: a. auxotrophic mutations b. somatic mutations c. morphological mutations d. oncogenes e. temperature sensitive mutations 3. What would be the frequency of AABBCC individuals from a mating of two AaBbCc individuals? a.. 1/64 b. 1/32 c. 1/16 d. 1/8 e. 3/16 f. 1/4 5. Polyploidy refers to: a. extra copies of a gene adjacent to each other on a chromosome b. an individual with complete extra sets of chromosomes c. a chromosome which has replicated but not divided d. multiple ribosomes present on a single mRNA e. an inversion which does not include the centromere 6. A gene showing codominance a. has both alleles independently expressed in the heterozygote b. has one allele dominant to the other c. has alleles tightly linked on the same chromosome d. has alleles expressed at the same time in development e. has alleles that are recessive to each other 7. The phenomenon of “independent assortment” refers to: a. expression at the same stage of development b. unlinked transmission of genes in crosses resulting from being located on different chromsomes, or far apart on the same chromosome. c. association of an RNA and a protein implying related function d. independent location of genes from each other in an interphase cell e. association of a protein and a DNA sequence implying related function 9. Which component of transcribed RNA in eukaryotes is present in the initial transcript but is removed before translation occurs: a. Intron b. 3’ Poly A tail c. Ribosome binding site d. 5’ cap e. codons coding for the protein to be produced 13. An Hfr strain of E. coli contains: a. a vector of yeast or bacterial origin which is used to make many copies of a particular DNA sequence b. a bacterial chromosome with a human gene inserted c. a bacterial chromosome with the F factor inserted d. a human chromosome with a transposable element inserted e. a bacterial chromosome with a phage inserted 14. An experiment was conducted in E. coli to map the following genes (pro, his, bio, met, phe and trp) on a circular map using 3 different Hfr strains. Strain 1 Order of transfer (early to late): trp met his pro Strain 2 Order of transfer (early to late): his met trp bio Strain 3 Order of transfer (early to late): pro phe bio trp Based on the results what is the most likely map? - D 15. Generation of antibody diversity in vertebrate animals takes place through: a. the presence of as many genes in the germ line as there are types of antibodies possible. b. infection with bacteria carrying antibody genes c. infection with viruses carrying antibody genes d. polyploidy in antibody-forming cells e. rearrangement of DNA in tissues that go on to produce antibodies 18. What is the co-transduction frequency for the A and B genes, from the following dataset? (Assume that there has been selection for the A+ form of the A gene). Genotype Number A+B+ C+ 10 A+B+ C30 A+ B- C+ 20 A+ B- C40 a. .10 b. .20 c. .30 d. .40 e. .50 21. Homeobox sequences a. are present in the genome of many animal species b. are found in prokaryotes but not in eukaryotes c. were identified as the integration sites for bacterial viruses d. represent integration sites for transposable elements e. represent the termination signals for transcription 23. Zinc finger proteins and helix-turn-helix proteins are: a. types of DNA-binding proteins b.involved in the control of translation c.components of ribosomes d.part of the hemoglobin in blood cells e.bound to transfer RNA during replication 24. Transcriptional activator proteins: a. transcribe a messenger off a DNA template b. bind to ribosomes to activate the production of specific proteins c. are produced during an infection of bacteria by a phage d. are essential to function of transfer RNAs during translation e. bind regions near a eukaryotic gene and allow an RNA polymerase to transcribe a gene 25.Differential distribution of substances in the egg most typically results in: a. differences in gene expression which may establish a pattern in the embryo as the cells divide b. amplification of specific genes during development c.development of polyploid tissues d.loss of specific genes during development e.dominance of genes derived from the father 27. A homeotic mutation is one which: a. is present in only one form in an individual b. substitutes one body part for another in development c. results in development of a tumor d. is wild type at one temperature and abnormal at another e. leads to increased body size in an organism 28. Assuming that the level of glucose is low, a mutation in the repressor of the lac operon in E. coli, preventing binding of the repressor to the operator, should result in: a. constitutive expression of the lac operon genes b. lack of expression or reduced expression of the lac operon genes under all circumstances c. expression of the genes only when lactose is present d. expression of the genes only when lactose is absent 29. Assuming that the level of glucose is low, a mutation in the repressor associated with the lac operon of E. coli which prevents binding of the repressor to lactose should result in: a. constitutive expression of the lac operon genes b. lack of expression or reduced expression of the lac operon genes under all circumstances c. expression of the genes only when lactose is present d. expression of the genes only when lactose is absent 30. RFLP analysis is a technique that a. uses hybridization to detect specific DNA restriction fragments in genomic DNA b. is used to determine whether a gene is transcribed in specific cells c. measures the transfer frequency of genes during conjugation d. is used to detect genetic variation at the protein level. e. is used to amplify genes for producing useful products 31.Plasmid vectors for cloning a. can generally accommodate larger inserts than phage vectors can b. grow within bacteria, and are present in bacterial colonies on an agar plate c. can accommodate inserts of over 100 kilobases d. include centromeres to allow propagation in yeast e. burst bacteria and form plaques on a “lawn” of bacteria 32.Simple tandem repeat polymorphisms in humans are most useful for: a. solving criminal and paternity cases b. reconstructing the relationships of humans and chimps. c. estimating relationships of humans and Neanderthals d. transferring disease resistance factors into bone marrow cells e. estimating matches for blood transfusions 33.The polymerase chain reaction or PCR is a technique that a. was used to demonstrate DNA as the genetic material b. is used to determine the content of minerals in a soil sample c. uses short DNA primers and a thermostable DNA polymerase to replicate specific DNA sequences in vitro. d. measures the ribosome transfer rate during translation e. detects the level of polymerases involved in replication 34.Positional cloning refers to: a. using a selection procedure to clone a cDNA b. cloning a portion of a gene using PCR c. isolating a gene by PCR using primers from another species d. isolating a gene from a specific tissue in which it is being expressed e. mapping a gene to a chromosomal region and then identifying and cloning a genomic copy of the gene from the region 36.On average, how many fragments would a restriction enzyme which recognizes a specific 4 base sequence in DNA be expected to cleave a double-stranded bacteriophage with a genome size of 5,000 bp into? a. about 2 b. about 4 c. about 20 d. about 50 e. about 1250 37.The “sticky ends” generated by restriction enzymes allow: a. selection for plasmids lacking antibiotic resistance b. easy identification of plasmids which carry an insert c. replication of transfer RNA within the bacterial cell d. insertion of centromeres into ribosomes lacking them e. pieces of DNA from different sources to hybridize to each other and to be joined together 38.QTL analysis is used to: a. identify RNA polymerase binding sites b. map genes in bacterial viruses c. determine which genes are expressed at a developmental stage d. identify chromosome regions associated with a complex trait in a genetic cross e. determine the most rapidly-evolving parts of genes 39.Assuming Hardy-Weinberg equilibrium, the genoypte frequency of heterozygotes, if the frequency of the two alleles at the gene being studied are 0.6 and 0.4, will be: a. 0.80 b. 0.64 c. 0.48 d. 0.32 e. 0.16 40.The likelihood of an individual in a population carrying two specific alleles of a human DNA marker, each of which has a frequency of 0.2, will be: a. 0.4 b. 0.32 c. 0.16 d. 0.08 e. 0.02 41.A threshold trait is one which: a. is expressed on a continuous scale (such as blood pressure) b. is present in a few discrete classes, but is influenced by both genetics and the environment (such as diabetes or schizophrenia) c. is caused by only a single gene, with no environmental influence d. is present in a very low frequency in the population e. is associated with superior survival of the heterozygote 42. Mitochondrial DNA is advantageous for evolutionary studies because: a. it is inherited only through the female parent and thus evolves in a way that allows trees of relationship to be easily constructed b. it is inserted into the X chromosome c. it first appeared in humans and is not found in other animals d. it evolves more slowly than the genes in the nucleus e. it was derived from the globin genes as an extra copy 49.If the frequency of males affected with an X-linked recessive condition in a human population is .10 (one in ten), what will be the expected frequency of affected females? a. 0.0001 b. 0.001 c. 0.02 d. 0.01 e. 0.05 Cell Bio!!!! 1. Which of the following statements concerning the composition of cell membranes is CORRECT? a) Plasma membranes contain lipid rafts that are rich in phospholipids with short fatty acid chains. b) Phosphatidylserine is found in high concentrations in the outer leaflet of the plasma membrane. c) Phospholipids in the plasma membrane can be covalently modified during cell signalling. d) The percentage of specific phospholipids is the same for all cellular membranes. e) Glycolipids are found in higher amounts in the cytosolic leaflet of the plasma membrane. 2. You would like to determine how a specific membrane protein is anchored to the plasma membrane. You express a fusion protein in cells in which the protein of interest is fused to GFP. As expected, most of the fluorescence is localized to the plasma membrane. You then add phospholipase C to the medium bathing the cells. The fluorescence disappears from the membrane. What can you conclude? a) The protein is anchored via its transmembrane region, which is composed of amino acids. b) The protein is a peripheral protein. c) The protein is anchored via a prenyl anchor. d) The protein is anchored via a glycosylphosphatidylinositol anchor. e) The protein is anchored via a fatty acid chain. 3. Which of the following is a step in the model of Na+/K+ pump action? a) The pump is not phosphorylated, instead the hydrolysis of ATP simply provides energy. b) In one conformation, 3 Na+ ions bind to high affinity sites on the extracellular face. c) Na+ ions dissociate from low affinity sites on the cytosolic face. d) Na+ ions and K+ ions are transported through the pump simultaneously. e) In one conformation, 2 K+ ions bind to high affinity sites on the exoplasmic face. 4. Glucose uniporters are a family of proteins that transport glucose. Which of the following concerning these uniporters is CORRECT? a) GLUT-2 has a high Km indicating that it has a high affinity for glucose. b) GLUT-4 is localized to plasma membranes of fat and muscle cells when insulin levels are low. c) GLUT-1 supplies glucose to most cells of the body independent of insulin concentrations. d) GLUT-1 has a low Km indicating that it has a low affinity for glucose. e) At low glucose concentrations, the rate of transport through GLUT-2 is faster than the rate of transport through GLUT-1. 5. Which of the following statements concerning ATP synthase and the synthesis of ATP is NOT correct? a) ATP synthase contains a transmembrane F0 region through which protons travel. b) The energy supplied as protons move down their electrochemical gradient is converted to mechanical energy. c) ATP synthases are of ancient origin and occur in plants, animals and bacteria. d) ADP and Pi bind to subunits in the F1 region where they are converted to ATP. e) V-type ATPases pump protons into the intermembrane space of mitochondria to generate a gradient that provides the energy for ATP synthesis. 6. Which of the following contributes most to the generation of the resting membrane potential in animal cells? a) Active transport of K+ into the cell by the Na+/K+ ATP pump. b) Passive transport of K+ out of cells via K+ leak channels. c) P-type ATPases that actively pump H+ out of cells. d) Active transport of Na+ into the cells by the Na+/K+ ATP pump. e) Passive transport of K+ out of cells via voltage-gated K+ channels. 7. Which of the following is (are) CORRECT concerning characteristics of antiporters? a) They transport two solutes in the same direction across the membrane. b) They transport one solute against and one solute with an electrochemical gradient. c) They transport one solute in one direction and another solute in the opposite direction across the membrane. d) both a and b. e) both b and c. 8. Which of the following statements concerning plant vacuoles is CORRECT? a) Cl- moves down its concentration gradient and into vacuoles via channel proteins. b) V-type ATPases pump protons out of vacuoles and into the cytoplasm. c) The energy required to transport Na+ into vacuoles via Na+/H+ antiporters is supplied by a proton motive force. d) The inside of the membrane of the vacuole is negatively charged in comparison with the cytosolic side of the membrane. e) P-type ATPases pump Ca2+ against its electrochemical gradient into the lumen of the vacuole. 9. Which of the following concerning the ABC transporter superfamily is CORRECT? a) Lac permease is an example of an ABC transporter. It pumps lactose out of bacterial cells and into the surrounding fluid. b) In eukaryotic cells, the main role of ABC transporters is to pump drugs into cells. c) Cystic fibrosis is caused by a mutation in an ABC transporter that functions as a chloride channel. d) Protists called Plasmodium falciparum cause malaria. Some P. falciparum have become resistant to drugs like chloroquine because they have developed a mutation that inhibits the activity of an ABC transporter. e) ABC transporters contain a GTP-binding domain. The transporter is active when it is bound to GTP, but inactive when it is bound to GDP. 10. Which of the following concerning the secretion of insulin from beta cells of the pancreas is CORRECT? a) Glucose is transported into beta cells via GLUT4 transporters. b) Acetylcholine binds to receptors on the beta cell and causes the closure of ATP-gated K+ channels. c) When glucose enters beta cells, one of its effects is to bind to voltage-gated Ca2+ channels, which causes them to open. d) Acetylcholine stimulates the synthesis of inositol trisphosphate, which binds to receptors in the endoplasmic reticulum. e) When ATP binds to ATP-gated K+ channels, the channels open and the membrane potential becomes more positive. 11. What is the function of Ran-GEF? a) It binds to nucleoporins ensuring that cargo is transported out of the nucleus. b) It promotes the exchange of GDP for GTP on Ran. c) It binds to the nuclear export signal of the cargo protein. d) It transports the empty nuclear export receptor into the nucleus. e) It enhances the intrinsic GTPase activity of Ran. 12. The gene coding for a soluble protein that is normally targeted to the lumen of peroxisomes is mutated so that an ER signal sequence is added to the encoded protein at the N-terminal end. The normal peroxisomal signalling sequence remains in the encoded protein. What is the most likely final destination of the protein? a) Lysosomes. b) The nucleus. c) The protein would be secreted from the cell. d) Peroxisomes. e) The Golgi apparatus. 13. Which of the following concerning the synthesis of type 1 transmembrane proteins is CORRECT? a) These proteins have several start transfer and stop transfer sequences and as a result span the membrane several times. b) In the cytosol, the SRP binds to the signal recognition sequence and protein synthesis continues during transit to the endoplasmic reticulum. c) The orientation of the protein is determined by the charge of amino acids found on either side of the stoptransfer sequence. d) The N-terminal signal recognition sequence is cleaved from the protein as it is synthesized in the endoplasmic reticulum. e) The region of the protein that binds to the SRP and the region of the protein that becomes the transmembrane domain is the same. 14. One of the reasons for glycosylation of proteins is to serve as a tag for unfolded proteins in the endoplasmic reticulum (ER). What is the role of glycosyl transferase in this process? a) It removes the 3 glucose residues that are found at the end of N-linked oligosaccharides. b) It is embedded in the membrane of the ER and binds to unfolded proteins, thereby preventing them from aggregating. c) It binds to unfolded proteins in the lumen of the ER and adds glucose molecules, which then bind to calnexin. d) It is the enzyme that adds a large preformed oligosaccharide onto asparagine residues in proteins. e) It glycosylates calnexin and calreticulin allowing them to interact and help to properly fold proteins that are unfolded. 15. Which of the following statements concerning clathrin-coated vesicles is CORRECT? a) Arf-1 is a GTPase that is needed for the formation of clathrin-coated vesicles. b) Clathrin-coated vesicles form in the membrane of the endoplasmic reticulum. c) Clathrin-coated vesicles lack V-SNARES. d) Uncoating of clathrin-coated vesicles requires a GAP protein. e) There is a single type of adaptor protein that interacts with clathrin. 16. Protein coats must be present on the vesicle membrane for: a) formation of a cage-like structure. b) docking of the vesicle. c) fusion of the vesicle with the target membrane. d) interaction of Rab-GTPases with Rab effectors. e) unravelling of V-SNARES and T-SNAREs. 17. New experimental evidence was presented in class that favours the cisternal model of protein transport through the Golgi apparatus. In one experiment, a cis-Golgi resident protein was labelled with GFP and a transGolgi resident protein was labelled with RFP. Single cisternae were observed over time with 3D confocal microscopy. The researchers observed that greencoloured cisternae changed into red-coloured cisternae. Why did the green disappear or the red appear? a) The red appeared because the RFP-labelled proteins were transported to the observed cisternae from the medial-Golgi. b) The green disappeared because the GFP-labelled proteins were transported out of the observed cisternae in an anterograde direction. c) The red appeared because the observed cisternae fused with the cisternae that contained the RFP-labelled proteins. d) The green disappeared because the GFP-labelled proteins were transported out of the observed cisternae in a retrograde direction. e) The green disappeared because the GFP-labelled proteins travelled through the Golgi and were secreted from the cell. 18. Which of the following is CORRECT? a) Lysosomal proteins are fully synthesized in the cytosol and then are targeted to the lysosome via their SKL sequence. b) Proteins that are targeted to the lysosome have an ER signal sequence. c) Lysosomes have a pH that is higher than that of the cytosol. d) Lysosomal proteins have a sequence of amino acids that binds to the mannose-6e) Proteins that are targeted to the lysosome have a KDEL sequence on their C-terminus. 19. The following experiment was performed to examine the unfolded protein response. One group of yeast cells was treated with a chemical that causes the unfolded protein response, while another group of cells remained untreated. RNA and protein were isolated from both groups of cells and northern and western blots were performed. The northern blot was probed with DNA that is complementary to an exon that is present in RNA coding for a gene regulatory protein that regulates the unfolded protein response. The western blot was probed with an antibody that is specific for the gene regulatory protein. Which of the following is the BEST answer for what you would expect to observe? a. Sample Untreated cells Treated cells Northern Band present Band present same weight as untreated Western No band Band present Sample Untreated cells Treated cells Northern Band present Band present smaller weight than untreated Western No band Band present Sample Untreated cells Treated cells Northern Band present Band present larger weight than untreated Western No band Band present Sample Untreated cells Treated cells Northern No band Band present Western Band present Band present smaller weight than untreated Sample Untreated cells Treated cells Northern No band Band present Western No band Band present b c d e 22. Which of the following concerning the cAMP second messenger signalling pathway is CORRECT? a) Adenylyl cyclase catalyses the conversion of cAMP to AMP so that the signal is downregulated. b) Phosphorylated CREB phosphorylates CBP proteins to help activate transcription of genes that have CREBresponse elements. c) Arrestin binds to G-protein coupled receptors to enhance the binding of Gα to the receptor, thereby activating Gα. d) Gα-GTP phosphorylates specific target proteins to produce biological effects in cells. e) A-kinase anchoring proteins bind to kinases, substrates and/or phosphatases to create focal points for signalling. 23. In receptor tyrosine kinase signalling involving the MAP kinase pathway, what is the step occurs immediately after the binding of Grb2 to the receptor? a) MAP-kinase goes to the nucleus. b) Receptor dimerization. c) Activation of Ras. d) Activation of a MAP-kinase-kinase-kinase. e) Activation of a guanine nucleotide exchange factor. 24. Skeletal muscle cells that have a functional insulin signalling pathway are treated with insulin. An hour later, a protein extract is made from the cells. Proteins in the extract are separated by SDS-PAGE and then the gel is blotted. The blot is incubated with antibodies that detect phosphoserine, phosphothreonine and phosphotyrosine residues in proteins. The blot is washed and then incubated with an appropriate secondary antibody to allow for detection of the primary antibodies. Which of the following proteins would you expect to detect on the blot? Give a complete list. 1. insulin receptor 2. IRS-1 3. Grb2 4. Phosphatidylinositol 3-kinase (PI 3-kinase) 5. PDK1 6. protein kinase B (PKB) a) 2, 3, 4 b) 1, 2, 3, 5, 6 c) 4, 5, 6 d) 1, 2, 5, 6 e) 2, 4 26. Which of the following statements concerning actin filament elongation is INCORRECT? a) Short oligomers can form spontaneously. b) The rate limiting step for filament elongation is filament nucleation. c) Cells can stabilize nucleation events to promote filament polymerization. d) Nucleation is slow without regulatory proteins. e) All actin filaments in cells are continually elongating. 27. During the growth phase of actin filaments in cells, which of the following statements is TRUE? a) Polymerization only depends on the rate constant of monomer addition and the monomer concentration. b) Polymerization occurs equally at both ends of the filament. c) The rate of monomer loss from the filament is independent of the free monomer concentration. d) The critical concentration is the concentration of free monomers that drives maximum formation of new filaments. e) The rate of monomer addition to the filament must always equal the rate of monomer loss from the filament. 28. Which of the following statements concerning microtubules is most accurate? a) Protofilaments containing tubulin-GDP are straighter than those containing tubulin-GTP. b) The effect of gamma-tubulin on microtubules is analogous to the effect of the Arp (Arp2/3) complex on actin filaments. c) Microtubules always maintain a cap of tubulin-GTP. d) The major function of microtubules is providing mechanical strength to cells. e) The plus ends of microtubules are bound to centrosomes. 29. Regarding intermediate filaments, which of the following statements is TRUE? a) Intermediate filaments composed of one type of protein are found in all cells. b) Intermediate filaments are named “intermediate” because their ability to withstand force and deformation is intermediate between that of microtubules and actin filaments. c) Keratin and collagen are two types of intermediate filaments. d) Some intermediate filaments can interact with adhesion complexes and others strengthen the cell nucleus. e) Intermediate filaments are polarized. 30. Which of the following statements concerning cell adhesion complexes is INCORRECT? a) Tight junctions are a type of communicating junction. b) Adherens junctions are a type of anchoring junction. c) Selectins form anchoring junctions. d) Claudins are a component of tight junctions. e) Connexons are a component of gap junctions. 31. The cadherin-cadherin binding mechanism is most accurately described by which of the ollowing sequence of events? a) (1) cis-dimerization promotes receptor straightening; (2) calcium promotes heterophilic, trans-interactions between the receptors; (3) the receptors interact with actin. b) (1) cis-dimerization promotes receptor straightening; (2) calcium promotes homophilic, trans-interactions between the receptors; (3) the receptors form large clusters. c) (1) Calcium promotes receptor straightening; (2) cis-dimerization promotes homophilic, trans-interactions between the receptors; (3) the receptors form large clusters. d) (1) Calcium promotes receptor straightening; (2) cis-dimerization promotes heterophilic, trans-interactions between the receptors; (3) the receptors form large clusters. e) (1) trans-interactions between the receptors promote cis-dimerization; (2) calcium promotes receptor clustering; (3) the receptors interact with actin. 33. Which of the following statements concerning the extracellular matrix is INCORRECT? a) A typical glycosaminoglycan molecule occupies a smaller volume than an actin molecule. b) Glycosaminoglycans are polysaccharides. c) Collagen and elastin both provide tensile strength. d) Overall, the extracellular matrix provides both tensile strength and resistance to compression. e) The basal lamina is thinner than connective tissue. 34. The extracellular matrix is primarily secreted by what type of cells? a) epithelial cells b) fibroblasts c) osteoblasts d) macrophages e) muscle cells 35. Which of the following molecules is a major link between the extracellular matrix and integrins? a) collagen b) actin c) elastin d) aggrecan e) laminin 36. Which of the following statements concerning focal contacts is most accurate? a) Integrin receptors mediate homophilic protein-protein interactions in the extracellular space. b) One type of alpha integrin subunit always interacts with the same type of beta subunit. c) The beta integrin subunit functions in focal contacts in the same way that beta catenin functions in adherens junctions. d) Focal adhesion function requires divalent cations. e) Focal adhesions do not interact with the cytoskeleton. 37. Individual cell migration is most accurately described by which of the following sequence of events? a) (1) Actin polymerization extends from focal contacts in the direction of migration; (2) New focal contacts are made and the treadmilling actin network engages them to continue the cell extension; (3) Actin depolymerization at the minus end of the treadmilling network detaches the cell from focal contacts at the rear. b) (1) Actin polymerization extends from focal contacts in the direction of migration; (2) New focal contacts are made and the treadmilling actin network engages them to continue the cell extension; (3) Myosin II contraction detaches the cell from focal contacts at the rear. c) (1) Actin polymerization extends the cell forward in the direction of migration; (2) Focal contacts are disassembled at the front of the cell to allow it to move over the substratum (3) Myosin II contraction also detaches the cell from focal contacts at the rear. d) (1) Actin polymerization extends the cell forward in the direction of migration; (2) Focal contacts are disassembled at the front of the cell to allow it to move over the substratum; (3) Actin depolymerization at the minus end of the treadmilling network also detaches the cell from focal contacts at the rear. e) (1) Individual actin filaments are stabilized by actin binding proteins; (2) Myosin II thick filaments move the actin filament past one another to extend the actin filament system forward; (3) As the actin filaments slide forward they engage focal contacts to pull the rest of the cell forward. 38. Which of the following complexes is most likely to act as a green light to progress though a cell cycle check point? a) non-phosphorylated cyclin E bound to phosphorylated Cdk2 b) phosphorylated cyclin E bound to non-phosphorylated Cdk2 c) non-phosphorylated cyclin E bound to non-phosphorylated Cdk2 d) phosphorylated cyclin E bound to phosphorylated CAK e) phosphorylated cyclin E bound to non-phosphorylated CAK 39. Which of the following would be expected to block a cell cycle checkpoint? a) Cdc25 phosphatase activity b) Inhibition of Wee1 kinase c) Degradation of p27 d) Synthesis of p27 e) Synthesis of cyclin E 40. Which of the following statements concerning Myc is INCORRECT? a) Myc regulates cyclins and Cdk inhibitor proteins. b) Myc is a tumour suppressor. c) Myc functions downstream of mitogen signaling pathways. d) Myc is a transcription factor. e) It is important to normally keep Myc levels relatively low. 41. How would you best describe the caspase signaling pathway? a) An inverted pyramid in which many separate signals at the top converge to elicit one final molecular effect. b) A single pathway in which a signal at the top elicits one final molecular effect in the nucleus. c) A single pathway in which a signal at the top elicits one final molecular effect in the mitochondria. d) A normal pyramid in which one initial signal at the top can elicit many separate molecular effects. e) A disorderly pathway associated with necrosis. 42. Which of the following statements concerning the control of cell numbers is most accurate? a) Cell apoptosis is unaffected by neighbouring cells. b) Cell division is unaffected by neighbouring cells. c) Apoptosis only occurs after acute cell damage. d) The formation of cell-cell contacts irreversibly blocks cell division. e) Cell-substratum adhesion can promote cell division. 43. The preparation of DNA for mitosis is most accurately described by which set of events? a) (1) During S phase, the chromatids assemble kinetochores to interact with microtubules; (2) During prophase, condensins compact the chromatids and cohesins hold the chromatids together; (3) DNA is then duplicated to form sister chromatids. b) (1) During S phase, condensins compact the chromatids and cohesins hold the chromatids together; (2) During prophase, the chromatids assemble kinetochores to interact with microtubules; (3) DNA is then duplicated to form sister chromatids. c) (1) During S phase, DNA is duplicated to form sister chromatids; (2) During prophase, cohesins compact the chromatids and condensins hold the chromatids together; (3) The chromatids then assemble kinetochores to interact with microtubules. d) (1) During S phase, cohesins compact the chromatids and condensins hold the chromatids together; (2) During prophase, the chromatids assemble kinetochores to interact with microtubules; (3) DNA is then duplicated to form sister chromatids. e) (1) During S phase, DNA is duplicated to form sister chromatids; (2) During prophase, condensins compact the chromatids and cohesins hold the chromatids together; (3) The chromatids then assemble kinetochores to interact with microtubules. 44. Which of the following statements concerning the mitotic spindle is INCORRECT? a) Astral microtubules extend towards the plasma membrane. b) Overlapping microtubules pull the centrosomes together. c) Kinetochore microtubules form between centrosomes and centromeres. d) The movement of chromosomes involves microtubule disassembly. e) A functional spindle involves both plus- and minus-end directed microtubule motor proteins. 45. Which of the following statements concerning cancer is INCORRECT? a) Muscle cell cancers are called sarcomas. b) Benign tumours remain in one location in the body. c) Genetic anomalies can be detected with a karyotype. d) Mutations in both oncogenes and tumour suppressor genes lead to cancer. e) Cancer arises from loss of function mutations in oncogenes. 46. Which of the following statements concerning tumour suppressor genes is most accurate? a) In a normal individual, mutation of one copy of a tumour suppressor gene leads to cancer. b) In a normal individual, the development of cancer requires mutation of one copy of a tumour suppressor gene in one cell plus the mutation of the other copy of the gene in another cell. c) In a normal individual, the development of cancer requires mutation of one copy of a tumour suppressor gene plus the mutation of the other copy of the gene in the same cell. d) In a normal individual, the development of cancer requires mutation of one copy of a tumour suppressor gene in one cell plus the mutation of the same copy of the gene in another cell. e) Mutations in tumour suppressor genes are not passed onto the offspring because the individual dies. 47. Which of the following are both embryonic germ layers? a) endoderm and epiderm b) epiderm and mesoderm c) ectoderm and mesoderm d) ectoderm and epiderm e) mesoderm and blastocoel 48. Cell intercalation is most commonly associated with which tissue rearrangement? a) invagination b) convergent extension c) epithelial-to-mesenchymal transition d) lateral inhibition e) ingression 49. Which of the following statements concerning embryonic organizers is most accurate? a) When removed from its original position in the embryo, an organizer loses its function. b) Organizers signal by contact inhibition. c) Morphogens secreted from organizers affect cell structure but not cell differentiation. d) The organizer is removed to allow the development of digits on vertebrate limbs. e) Cells have graded responses to organizers. 50. Which of the following statements concerning homeotic (Hox) genes is INCORRECT? a) A Hox gene taken from one organism can function in another organism. b) The order of Hox gene expression along the embryo matches the order of Hox genes on the chromosome. c) Mutations in Hox genes can create flies with legs where their antennae should be. d) Hox genes encode cell surface receptors. e) Antennapedia is a Hox gene. 55. One of the in vitro muscle systems required the addition of calcium ions. What is the critical role of calcium with respect to muscle contraction? a) Calcium is a necessary co-factor for ATPase activity of the myosin head. b) Calcium binds to tropomyosin, which sends a signal to troponin to pull tropomyosin out of its actin binding groove. c) Calcium allows for the proper dynamics of the large titin protein. d) Calcium binds to troponin, which releases tropomyosin into its normal binding groove so that the myosin heads can bind. e) Calcium activates calmodulin, which sends a signal to the troponin complex to pull tropomyosin out of its actin binding groove, so the actin can bind to the myosin heads. 58. Which of the following proteins is NOT a component seen in the sliding filament contraction model? a) Titin b) Nebulin c) Cadherin d) Myosin e) CapZ 59. Which type of regular light microscopy is the BEST for visualizing transparent internal structures in living cells? a) Bright-field microscopy. b) Transmission electron microscopy. c) Nomarski Imaging (Differential Interference Contrast). d) Fluorescence microscopy. e) Confocal microscopy. 61. Which of the following is NOT an advantage of using photoactivatable GFP (PAGFP) compared with conventional GFP? a) Protein turnover can be measured. b) Specific pools of proteins within the cell can be measured. c) Proteins made with PAGFP are resistant to degradation and remain fluorescent throughout the life of a cell. d) Photoactivation takes a short time and therefore can be used instead of photobleaching to study diffusional mobility of proteins. e) PAGFP can be used as an expression marker to study subsets of cells within large populations of cells. 62. Which of the following is a CORRECT reason why a particular type of cellular component will fluoresce when FDA is added? a) The FDA is excited by the UV light emitted by adjacent cellular organelles. b) Enzymes with esterase activity are present within a type of cellular organelle. c) FDA is one component of fluorescence resonance energy transfer (FRET) and will fluoresce when excited with UV light. d) Enzymes with peroxidase activity release free radicals which will cause FDA to fluoresce. e) When cells are dead, FDA will cause particular cellular organelles to fluoresce. 65. What is the BEST description of the function of the condenser iris diaphragm on a light microscope? The condenser iris diaphragm may be used to: a) adjust the binocular vision until the left and right fields of view coincide. b) switch between regular light microscopy and phase contrast microscopy. c) exclude extraneous light and improve image contrast. d) move the condenser up and down to focus light on the specimen. e) obtain better resolution and contrast, while increasing depth of focus. 66. Which of the following statements about electron microscopy is TRUE? a) Scanning electron microscopy (SEM) is generally used to view internal cellular structures. b) The wavelength of a beam of electrons is about 1000 times shorter than that of visible light. c) Glass lenses are used to focus the electron beam. d) The achievable resolution of electron microscopy is similar to that of light microscopy. e) Transmission electron microscopy (TEM) can be combined with the metal shadowing process to view external cellular structures. 68. Which of the following statements about Drosophila is TRUE? a) mRNA and proteins in stage 10 follicles are distributed with bilateral symmetry. b) Embryonic germ cells give rise to the head of the adult fly. c) Peroxisomes transport mRNA and proteins to different areas of the oocyte. d) The polarity of the oocyte determines the polarity of the embryo. e) The dorsal appendage on the embryo was the location of sperm entry into the oocyte. 1. A parent cell divides to form two genetically identical daughter cells in the nuclear process of mitosis. For mitosis to take place a. the parent cell must first be fertilized. b. the parent cell must replicate all its chromosomes prior to mitosis c. the parent cell must replicate its DNA during telophase d. the parent cell must divide its DNA in half so that each daughter cells gets only the genetic information it needs e. the parent cell must stop all metabolic functions before entering the interphase that precedes mitosis 2. At the end of mitosis, each daughter cell has a. DNA that is identical to that of the parent cell b. twice the DNA and half the cytoplasm of the parent cell c. twice the cytoplasm and the same amount of DNA as the parent cell d. half the DNA and half the cytoplasm of the parent cell e. a new combination of chromosomes when compared to the parent cell 3. A centromere is a region in which a. chromatids are attached to one another b. metaphase chromosomes become aligned c. chromosomes are grouped during telophase d. the nucleus is located prior to mitosis e. new spindle microtubules form 4. The microtubules of the mitotic spindle attach to a specialized structure in the chromosome called the a. kinetochore b. centriole c. metaphase plate d. aster e. centrosome 5. A certain species of animal has six pairs of chromosomes. How many molecules of DNA do the nuclei of this animal have in the G2 phase? a. 3 b. 6 c. 12 d. 24 e. 48 6. In animals, cytokinesis occurs when a. microtubules pull the cell membrane into a cleavage furrow b. immediately before mitosis c. when vesicles from the Golgi body build up to divide the cell in two d. when G2 is complete e. when a ring of actin and myosin filaments (microfilaments) contracts 7. The first gap in the cell cycle (G1) corresponds to a. normal growth and functioning b. the period in which DNA is being replicated c. the beginning of mitosis d. the stage between DNA replication and the M phase e. the stage the cell enters when it loses its ability to divide 8. The mitotic spindle is a microtubular structure that is involved in a. splitting of centromeres in anaphase b. triggering the condensation of chromosomes c. disassembly of the nucleolus d. fragmentation of the nuclear envelope e. separation of sister chromatids 9. Metaphase is characterized by a. aligning of chromosomes on the metaphase plate b. splitting of centromeres c. cytokinesis d. disassembly of the nuclear envelope e. a doubling in the number of chromosomes 10. When dividing cells are examined under a light microscope, chromosomes first become visible during a. interphase b. the S phase c. prophase d. G1 e. G2 11. The M-phase checkpoint is designed to make sure all chromosomes are attached to the mitotic spindle. In which stage of mitosis would those cells be most likely to arrest? a. interphase b. telophase c. prophase d. prometaphase e. metaphase 12. If a cell has accumulated DNA damage, it is unlikely to a. pass the G2 checkpoint b. activate DNA repair enzymes c. enter G1 from mitosis d. synthesize cyclin-dependent kinases e. go through apoptosis The following questions consist of phrases or sentences related to the control of cell division. For each one select the term below that is most closely related to the statement. Each term may be used once, more than once, or not at all. a. PDGF b. MPF c. Protein kinase d. Cyclin e. CdK 13. One of the external signals involved in regulating the cell cycle. It is released in the vicinity of an injury a b c d e 14. A general term for enzymes that activate or inactivate other proteins by phosphorylating them a b c d e 15. A protein maintained at constant levels throughout the cell cycle that requires cyclin to become catalytically active a b c d e 16. Triggers the cell’s passage past the G2 checkpoint into mitosis a b c d 17. One difference between a cancer cell and a normal cell is that a. the cancer cell is unable to synthesize DNA b. the cell cycle of the cancer cell is arrested at the S phase c. cancer cells continue to divide even when they are tightly packed together d. cancer cells cannot function properly because they suffer density-dependent inhibition e. cancer cells are always in the M-phase of the cell cycle 18. Assume that a particular human cell is examined under a microscope and it contains 22 autosomic chromosomes and a Y chromosome. The cell is most likely to be a. a somatic cell of a male b. a somatic cell of an individual suffering from Downs’s syndrome c. a fertilized egg (a zygote) d. a somatic cell of a female e. a sperm 19. Homologous chromosomes move to opposite poles of a dividing cell during a. mitosis b. meiosis I c. meiosis II d. fertilization e. none of the above 20. The DNA content of a diploid cell in the G1 phase of a meiotic interphase is X. The DNA content of a meiotic metaphase II cell from the same organism would be a. 0.25 X b. 0.5 X c. X d. 2 X e. 4 X 21. If a typical diploid somatic cell has 32 chromosomes, how many chromosomes are expected in each gamete of that organism? a. 32 b. 64 c. 16 d. 0 e. 46 22. At which stage of meiosis is the chromosome number typically reduced from 2n to n a. meiosis II b. metaphase II c. interphase d. mitosis e. meiosis I 23. Meiosis II is similar to mitosis in that a. homologous chromosomes synapse b. DNA replicates immediately before the division c. the daughter cells are diploid d. sister chromatids separate during anaphase e. the chromosome number is reduced 24. Crossing-over usually contributes to genetic variation by exchanging chromosomal segments between a. sister chromatids of a chromosome b. chromatids of non-homologous chromosomes c. non-sister chromatids of homologous chromosomes d. non-homologous chromosomes e. autosomes and sex chromosomes 25. What is a tetrad? a. the “X” that forms when crossing-over occurs b. a group of four chromatids produced when homologs synapse c. the four points where homologous chromosomes touch as they synapse d. the group of four genetically identical daughter cells produced by mitosis e. the group of four haploid cells produced by meiosis 26. Meiosis can occur a. in all organisms b. only when an organism is diploid c. only in multicellular organisms d. only in haploid organisms e. only in single-celled organisms 27. At the end of meiosis I, each chromosome consist of a. a homologous chromosome pair b. four copies of a DNA molecule c. one copy of a DNA molecule d. two chromatids e. a pair of polar microtubules 28. What is a karyotype? a. the set of unique physical characteristics that define an individual b. the collection of all mutations present within a genome c. a unique combination of chromosomes found in a gamete d. a system of classifying cell nuclei e. a display of every pair of homologous chromosomes within a cell organized according to size and shape 29. At which stage of mitosis are chromosomes photographed in the preparation of a karyotype? a. prophase b. metaphase c. anaphase d. telophase e. interphase 30. Crossing-over occurs during which phase of meiosis? a. prophase I b. anaphase I c. telophase I d. prophase II e. metaphase II 31. In 1928, Griffith injected S (smooth) bacteria into mice, and the mice died. When he injected living R (rough) bacteria into mice, the mice lived. When he injected heat-killed smooth bacteria into mice, the mice lived. What was the result when he mixed heat-killed S bacteria with live R bacteria and injected this mixture into mice? a. the mice lived b. the mice died indicating that heat-killed S bacteria came back to life c. the mice lived, and living S bacteria could be obtained from the tissues of living mice d. the mice died, indicating that DNA replicated semi-conservatively e. the mice died, and living S cells could be isolated from the tissues of the dead mice 32. Assume that you have determined the percentages of bases in DNA samples from variety of organisms, each having DNA in the usual double-stranded form. What relationship would you expect to find in the percentage data? a. A = C b. A = G c. C = T d. A + C = G + T e. A + T = G + C 33. DNA replication is said to be semi-conservative because a. half of the DNA in a new cell comes from one gamete and other half from the other b. the same process of DNA replication is used by all organisms c. the number of nucleotides within genes remain constant d. each DNA molecule is composed of one old strand and one new strand e. the total amount of DNA within an individual remains the same 34. Where and how are Okasaki fragments synthesized? a. at the leading strand, oriented in a 5’ to 3’ direction b. at the leading strand, oriented in a 3’ to 5’ direction c. at the lagging strand, oriented in a 5’ to 3’ direction d. at the lagging strand, oriented in a 3’ to 5’ direction e. none of the above 35. Once DNA strands are separated by DNA Helicase, ____________ block the reformation of a double helix by the template strands a. RNA primer strands b. topoisomerase c. single-strand binding proteins d. RNA polymerase complexes e. DNA polymerase III 36. All of the following can be determined directly from X-ray diffraction photographs of crystallized DNA except the a. diameter of the helix b. sequences of nucleotides c. spacing of the bases along the helix d. number of strands in the helix e. helical shape of DNA 37. What kind of chemical bond is found between paired bases of the DNA double helix? a. hydrogen b. ionic c. covalent d. sulfhydryl e. phosphate 38. Suppose one were provided with an actively dividing culture of E. coli bacteria to which radioactive thymine has been added. What would happen if a cell replicated once in the presence of this radioactive base? a. One of the daughter cells but not the other will have radioactive DNA b. Neither of the two daughter cells will be radioactive c. All four bases of the DNA would be radioactive d. Radioactive thymine would pair with non-radioactive guanine e. DNA in both daughter cells will be radioactive 39. What determines the nucleotide sequence of the newly synthesize strand during DNA replication? a. the particular DNA polymerase catalyzing the reaction b. the relative amounts of the four nucleotides in the cell c. the nucleotide sequence of the template strand d. the primase used in the reaction e. the leading strand 40. What is the role of DNA ligase in the elongation of the lagging strand during DNA replication? a. synthesize RNA nucleotides to make primer b. catalyze the lengthening of telomeres c. join Okasaki fragments together d. unwind the parental double helix e. stabilize the unwound parental DNA 41. In eukaryotic cells, each chromosome has a. one origin of replication b. two origins of replication c. many origins of replication d. only one origin of replication per nucleus e. none of the above 42. The enzyme that removes the RNA primers is called a. DNA ligase b. primase c. helicase d. Topoisomerase e. DNA polymerase I 43. The 3’ end of a DNA strand is the place in the molecule where a. the phosphate group is not bound to another nucleotide b. both DNA strands end opposite each other c. polymerase builds the primer e. three adenine nucleotides are present 44. A codon a. consist of two nucleotides b. may code for the same amino acid as another codon c. consists of amino acids d. catalyzes RNA synthesis e. is found in all eukaryotes but not in prokaryotes 45. If the triplet CCC codes for the amino acid proline in bacteria, then in plants CCC should code for a. leucine b. valine c. cystine d. phenyl amine e. proline 46. RNA polymerase II uses the ______ DNA template to synthesize a ______ mRNA a. 5’ → 3’, 5 → 3’ b. 3’ → 5’, 3’ → 5’ c. 3’ → 5’, 5’ → 3’ d. 5’ → 3’, 3’ → 5’ e. example of all these have been found 47. Transcriptions is the process of a. synthesizing a DNA molecule from an RNA template b. assembling ribonucleotides into an RNA molecule without a template c. synthesizing an RNA molecule using a DNA template d. synthesizing a protein using information from a messenger RNA e. replicating a single-stranded DNA molecule 48. A transcription start signal is called a (an) a. initiation codon b. promoter c. origin d. operator e. nonsense codon 49. Which of the following is a post-transcriptional modification of mRNA found in eukaryotes? a. a 5’ cap b. a 3’ cap c. a poly T tail d. polyadenylation of the 5’ end e. none of the above 50. Exons are a. spliced out of the primary (original) transcript b. spliced together from the original transcript c. spliced to introns to form the final transcript d. much larger than introns e. larger than the original coding region 51. Transcription factors are a. RNA sequences that bind to RNA polymerase b. DNA sequences that regulate up transcription c. proteins that bind to DNA near the promoter sequence d. polysaccharides that bind to the transcripts e. enzymes that splice exons and introns 52. Eukaryotic protein-coding genes differ from their prokaryotic counterparts in that only eukaryotic genes a. are double-stranded b. are present in only a single copy c. contain introns d. have a promoter e. are transcribed into mRNA 28) Which organelle is primarily involved in the synthesis of phospholipids and steroids? A) ribosome B) chloroplast C) Golgi apparatus D) smooth endoplasmic reticulum (ER) E) nuclear envelope 33) Which of the following is present in a prokaryotic cell? A) mitrochondria B) ribosome C) nuclear envelope D) chloroplast E) endoplasmic reticulum 44) The light reactions of photosynthesis occur in what structure of the chloroplast? A) thylakoid membrane B) chlorophyll molecule C) stroma of the chloroplast D) cytoplasm surrounding the chloroplast E) outer membrane of the chloroplast 46) What is the primary function of the Calvin cycle? A) use ATP to release carbon dioxide B) use NADPH to release carbon dioxide C) split water and release oxygen D) transport Rubisco out of the chloroplast E) synthesize sugars from carbon dioxide 50) A cell containing 92 chromatids at metaphase of mitosis would, at its completion, produce two nuclei each containing how many chromosomes? A) 12 B) 23 C) 46 D) 92 E) 184 The lettered circles below show a diploid nucleus with four chromosomes. There are two pairs of homologous chromosomes, one long and the other short. One haploid set is symbolized as black and the other haploid set is gray. The chromosomes in the unlettered circle (at top) have not yet replicated. Choose the correct chromosomal conditions for each of the following stages in questions 52 to 54. 52) At prometaphase of mitosis A) A B) B C) C D) D E) E 53) One daughter cell nucleus at teleophase of mitosis A) A B) B C) C D) D E) E 54) One daughter cell nucleus at prophase of meiosis II A) A B) B C) C D) D E) E 55) Which of the following are primarily responsible for cytokinesis in plant cells? A) chromatid condensation B) Golgi-derived vesicles C) actin and myosin D) centrioles and basal bodies E) cyclin-dependent kinases 70) DNA polymerase III moves in which direction along the DNA? A) 3´ → 5´ along the template strand B) 3´ → 5´ along the coding (sense) strand C) 5´ → 3´ along the template strand D) 3´ → 5´ along the coding strand E) 5´ → 3´ along the double-stranded DNA 72) Why are viruses referred to as obligate parasites? A) They cannot reproduce outside of a host cell B) Viral DNA always inserts itself into host DNA C) They invariably kill any cell they infect D) They can incorporate nucleic acids from other viruses E) They must use enzymes encoded by the virus itself 73) What is the name given to viruses that have single-stranded RNA that acts as a template for DNA synthesis in the host cell? A) retroviruses B) proviruses C) bacteriophages D) prions E) phages MATCH!!!!!! The next five questions are MATCHING questions using the foils (A-E) below. Some foils may be used more than once or not at all. A. GMP B. UMP C. ATP D. dTMP E. dATP 51) Inhibits synthesis of PRPP (5-phosphoribosyl-1-pyrophosphate) from ribose 5-phosphate A 52) Directly stimulates the overall activity of ribonucleotide reductase - c 53) 5-fluorouracil is the chemotherapeutic drug used to target the last enzyme in the synthesis of this Nucleotide - D 54) Activates methyl group of methionine - C 55) Committed step in de novo synthesis of this nucleoside monophosphate requires PRPP. - A FILL IN THE BLANK (5 points each) 417. Answer the following questions by choosing items from the list below and placing the appropriate letter in the blank space. (One letter per blank, use each letter only once) a. UV light f. Thymine dimer b. Intercalating dyes g. Nonsense base substitution c. Tautomeric shifts h. Ionizing radiation d. Breaks in DNA backbone i. Tandem duplication e. Base analog chemicals j. Depurination A. Which kind of mutation cannot readily be induced? _c_________ B. Which kind of mutagenizing agent is most likely ___b_______ to cause frameshift mutations? C. Which kind of mutation can be completely ___f_______ reversed by photoreactivation? D. Which kind of mutation is most likely to be ___i_______ benificial for an organism? E. Which kind of mutagenizing agent is most likely ___d_______ to cause severe damage to the DNA backbone? 418. Match the phrases about gene regulation in prokaryotes to the terms in the list by putting a single letter in each blank. Not all letters need to be used, and a particular letter may be used more than once. a. Positive control f. Constitutive b. Activator g. Operator c. Negative control h. Repressor d. Inducible i. Effector molecule e. Noninducible j. Operon 1. Small molecule that binds to a regulatory protein to ____i_________ change its action 2. Binding site for regulatory proteins ______g_______ 3. Regulatory molecule that binds to operator region in DNA ____h_________ 4. Regulatory protein that promotes transcription ______b_______ Questions 62-73. Match the following techniques/equipment with a possible use. Each numbered question will have only one answer. Some answers may be used more than once, others not all. Use capital letters. A. Electrophoresis B. Centrifugation C. Light microscope D. Scanning electron microscope E. Transmission electron microscope F.Metric ruler G. Spectrophotometer H. Paper chromatography I. Answer not given 62. Examining the surface of cells. D 63. Looking at the internal structure of chloroplasts. E 64. Watching cells with flagella move. C 65. Determining the speed of snails. F 66. Separating proteins based on charge. A 67. Determining how much of a colored product is produced. G 68. Separating pigments of a leaf. H 69. Determining how much water a plant needs. I 70. Determining which kinds of cell have the largest number of lysosomes. E 71. Isolating nuclei from cells. B 72. Measuring the amount of gas produced by an enzyme. I 73. Separating different organelles in a cell. B 80-86. Match the process with its function. Some processes may be used more than once, others not all. Each function will have only one answer. Use capital letters. A. Light dependent reactions B. Light independent reactions C. Glycolysis D. Krebs cycle E. Electron transport system 80. Make ATP from light. A 81. Breakdown glucose into smaller carbon compounds. C 82. Make sugar from CO2. B 83. Make ATP and water. E 84. Make ATP and split water. A 85. Remove H+ from carbon compounds. D 86. Produce pyruvic acid. C **107-108. Cells of an onion root tip were examined and the following cells in various stages were reported: Interphase 25 Prophase 8 Metaphase 4 Anaphase 3 Telophase 1 Total 40 **107. What approximate % of cells is not dividing? . 15 A. 35 B. 45 C. 55 D. 65 **108. If mitosis in onion cells is 80 minutes long, how long is prophase? A. 20 min B. 30 min C. 40 min D. 50 min E. 60 min Questions 12 through 14 apply to the following clinical case: A person is brought into the emergency room suffering from incoordination, impaired reflexes, respiratory distress and central nervous system depression. You quickly conclude that the patient has been poisoned. Working with the local police you learn that the patient’s wife is a noted biochemist. You suspect that she used her expertise to deduce she could get rid of her husband by drastically increasing his dose of Lipitor, a cholesterol lowering drug. You realize that this would lead to an almost complete loss of CoQ (also known as CoQ10 and ubiquinol) and ultimately lead to complete mitochondrial dysfunction. 12) Given what you know about the electron transport chain, the loss of CoQ would limit the ability of which complex or complexes to donate their reducing equivalents to the electron transport chain? A) Complex I B) Complex II C) Complex III D) A and B E) All of the above 13) If you were able to isolate the mitochondria of the poisoned patient and measure their oxygen consumption rates before (compared to a healthy sample) and after the addition 2,4 dinitrophenol (2,4 DNP, compared to no 2,4 DNP) what would you observe? O2 consumption O2 consumption without 2,4 DNP Presence of 2,4 DNP Compared to healthy sample compared to sample before 2,4 DNP addition A) Near normal unchanged B) Near normal increased C) Suppressed unchanged D) Suppressed increased E) Increased unchanged 14) Given the relationship between the function of the electron transport chain and the utilization of reducing equivalents, what glycolytic enzyme would most directly be affected by mitochondrial dysfunction caused by the loss of CoQ? A) Phosphofructokinase 1 B) Glyceraldehyde 3-phosphate dehydrogenase C) Phosphoglycerate kinase D) Glucokinase E) Enolase