MEch CPR ev profile table Jan 22 2015 updated
advertisement

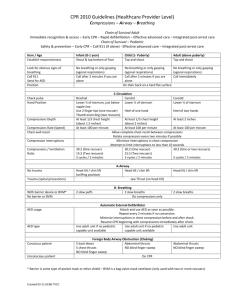
Author(s): Steven C Brooks, Laurie J Morrison Date: Question: Mechanical chest compressions compared to manual chest compressions for cardiac arrest Quality assessment № of studies Study design Risk of bias Inconsistency Indirectness № of patients Imprecision Other considerations Mechanical chest compressions n/N (%) manual chest compressions n/N (5%) Effect Relative Risk (95% CI) Quality Importance Absolute (95% CI) Survival to hospital discharge with good neurological function (assessed with: CPC scores or MRS ) 3 randomised trials serious 1 not serious not serious not serious none not pooled ⨁⨁⨁ ◯ CRITICAL MODERATE Hallstrom 2006 12/391 (3.07) 28/371 (7.55) 0.41 (0.210.79) Wik 2014 87/2099 (4.14) 112/2132 (5.25) 0.79 (0.601.03 108/1300 (8.31) 100/1289 (7.76) 1.07 (0.831.39) 105/1300 (8.1%) 94/1289 (7.3%) Rubertsson 2013 Survival to 30 days with good neurological outcome (assessed with: CPC scores) 1 randomised trials serious 2 not serious not serious not serious none Rubertsson 2013 1.11 (0.841.45) ⨁⨁⨁ ◯ CRITICAL MODERATE Survival to 60 days with good neurological function (assessed with: CPC scores) 0 see comment not pooled 98/1289 (7.6%) 1.11 (0.861.45) see comment CRITICAL Survival to 180 days with good neurological outcome 1 randomised trials serious 2 not serious not serious not serious none 110/1300 (8.5%) Rubertsson 2013 ⨁⨁⨁ ◯ CRITICAL MODERATE Survival to 1 year with good neurological outcome 0 Survival to hospital discharge see comment not pooled see comment CRITICAL Quality assessment № of studies Study design Risk of bias Inconsistency Indirectness № of patients Imprecision Other considerations Mechanical chest compressions n/N (%) manual chest compressions n/N (5%) Effect Relative Risk (95% CI) Quality Importance Absolute (95% CI) Survival to hospital discharge with good neurologic outcome (GCS 1-2 or mRS 0-3) 5 randomised trials serious 34 not serious 5 not serious not serious none not pooled ⨁⨁⨁ ◯ CRITICAL MODERATE Hallstrom 2006 23/394 (5.84) 37/373 (9.92) 0.59 (0.360.97) Lu 2010 25/76 (32.89) 11/74 (14.86) 2.21 (1.174.17) Rubertsson 2013 117/1300 (9) 118/1289 (9.15) 0.98 (0.771.25) 6/75 (8) 7/72 (9.72) 0.82 (0.292.33) 196/2099 (9.34) 233/2132 (10.93) 0.85 (0.711.02 Smekal 2011 Wik 2014 Survival to 30 days 2 randomised trials serious 27 not serious not serious not serious none Not pooled ⨁⨁⨁ ◯ CRITICAL MODERATE Perkins 2014 Rubertsson 2013 104/1652 (6.3) 193/2819 (6.85) 0.92 (0.731.16) 112/1300 (8.62) 109/1289 (8.07) 1.02 (0.791.31) see comment not pooled see comment 104/1289 (8.1%) not estimable not estimable Survival to 60 days 0 CRITICAL Survival to 180 days 1 Rubertsson randomised trials serious 2 not serious not serious not serious none 111/1300 (8.5%) ⨁⨁⨁ ◯ CRITICAL Quality assessment № of studies Study design Risk of bias Inconsistency № of patients Indirectness Imprecision Other considerations Mechanical chest compressions n/N (%) manual chest compressions n/N (5%) Effect Relative Risk (95% CI) Quality Importance Absolute (95% CI) 2013 MODERATE Survival to 1 year 1 randomised trials serious 7 not serious not serious not serious none 89/1652 (5.4%) 175/2819 (6.2%) not estimable not estimable Perkins 2014 ⨁⨁⨁ ◯ CRITICAL MODERATE Return of spontaneous circulation 7 randomised trials serious 137 serious 6 not serious 7 not serious none not pooled see comment ⨁⨁◯ ◯ IMPORTANT LOW Dickinson 1998 1/7 (14.29) 0/10 (0) Halperin 1993 8/17 (47.06 3/17 (17.65) 2.67 (0.858.37) 42/76 (55.26) 28/74 (37.84) 1.46 (1.022.08) 446/1289 (34.60) 1.02 (0.921.14) 23/72 (31.94) 1.27 (0.821.96) Lu 2010 460/1300 (35.38) Rubertsson 2013 30/74 (40.54) Smekal 2011 n/a Wik 2014 600/2099 (28.59) 689/ 2132 (32.32) 0.88 (0.810.97) Perkins 2014 522/1652 (31.60) 885/2819 (31.39) 1.01 (0.921.10) MD – mean difference, RR – relative risk 1. Problems with allocation concealment, blinding of outcome assesors. Hallstrom study: 99.3% of participants entered into the study had full follow-up data reported. Cointervention bias: Site C of the study changed the protocol halfway through the study; this involved applying the device to participants after a period of CPR and rhythm analysis. This change in CPR technique is likely to have had an impact on outcomes for participants treated at this site with mechanical CPR. Rubertsson study: 1. Control arm follows 2005 resuscitation guidelines. Outdated comparator. 2. Co-intervention: Treatment arm includes a novel study-specific protocol including defibrillation without pulse/rhythm checks not scientifically validated and may have an impact on the outcomes measured independent of the mechanical CPR intervention. 3. CPR quality measured in only 10% of the sample. This makes it difficult to interpret the nature of the control group and whether this was a valid control reflective of state-ofthe-art contemporary care. 4. 5% of enrolled patients had a protocol violation related to the inclusion or exclusion criteria but were included in the intension-to-treat analysis. 5. 5% of patients had some type of contamination (i.e. received treatment for which they were not allocated). 2. 3. 4. 5. 6. 7. In the Rubertsson study -1. Control arm follows 2005 resuscitation guidelines. Outdated comparator. 2. Co-intervention: Treatment arm includes a novel study-specific protocol including defibrillation without pulse/rhythm checks not scientifically validated and may have an impact on the outcomes measured independent of the mechanical CPR intervention. 3. No measure of quality of manual CPR in either group. This makes it difficult to interpret the nature of the control group and whether this was a valid control reflective of state-of-the-art contemporary care. 4.. The 4-hour end-point lacks rationale for the being the primary outcome. It is not a commonly excepted outcome and not validated in other prehospital arrest trials. 5. 5% of enrolled patients had a protocol violation related to the inclusion or exclusion criteria but were included in the intension-to-treat analysis. 6. 5% of patients had some type of contamination (i.e. received treatment for which they were not allocated). Issues of co-intervention with novel and unconventional CPR sequences in the mechanical chest compression arms . Blinding of the providers and patients not possible given the nature of intervention. Outcome assessors were generally not blinded. Dickinson study: Quasi-randomisation based on odd/even days. Quasi-randomisation did not allow for allocation concealment. Co-intervention issue: Both groups of participants placed on Thumper back-board. HCPR (control) group had machine running but not applied to participant to allow human compressions to be delivered at the same rate. Halperin study: Allocation sequence concealment: Sequenced envelopes used. Unclear whether these envelopes were opaque; no mention of a randomisation log to track any subterfuge of the randomisation process. Although there was considerable inconsistency in the direction of results from included studies when considering individual study results, the inconsistency was generated mostly by inclusion of smaller studies and studies with recognized sources of bias. The larger studies, Wik and Rubertsson demonstrate equivalence and no difference, respectively. The Hallstrom study demonstrated harm but has methods issues that likely contributed to this (see explanation 1) Dickinson, Halperin and Lu suggest benefit, Rubersson, Smekal, Wik and Perkins do not observe a difference. In the Perkins study - 1. 60 % of the intervention group did not receive any mechanical chest compressions. 2. No CPR quality measures were instituted. 3. Unclear if training and quality assurance was sufficient for EMS crews using the mechanical device and for manual CPR in control group.