AP Chemistry Chapter 10 Notes Remember from Chemistry Class

AP Chemistry
Chapter 10 Notes
Remember from Chemistry Class:
Intramolecular forces: ionic, metallic, covalent bonds (chapters 8 and 9)
much stronger than IM forces
Intermolecular (IM) forces: (see flow chart)
when substances change state, molecules remain intact, these forces are broken
Are polar molecules involved ?
Are H atoms bonded to N, O or F?
London dispersion forces only
Hydrogen bonding dipole-dipole forces examples:
Ar, I
2 examples:
H
2
O, NH
3
HF
, examples:
H
2
S, CH
3
Cl
Label each picture according to the type of IM force it illustrates:
According to the KMT, what three assumptions can we make?
1.
particles in constant, rapid, random motion (neglect intermolecular forces)
2.
particles have no volume, exist as “point masses”
3.
collisions are perfectly elastic (energy is perfectly conserved)
gases: weakest IM forces, particles move independently of one another
liquids: stronger IM forces but allowed to flow
(gases and liquids are fluids, the particles slip easily by one another)
solids: strongest IM forces, particles vibrate around fixed points
Why do noble gases have such low melting points?
LIQUIDS:
Some properties of liquids compared to gases:
low compressability
lack of rigidity
high density
IM forces will cause them to bead as droplets (surface tension)
(a sphere has maximum volume for minimum surface area)
Polar liquids will exhibit
capillary action: spontaneous rising of a liquid in a narrow tube.
cohesive forces (IM forces among molecules)
adhesive forces (IM forces between liquid molecules and their container - glass)
water (polar molecule) has a CONCAVE meniscus because the adhesive forces are stronger than the cohesive forces
non-polar molecules will have a CONVEX meniscus in a graduated cylinder
viscosity: resistance to flow glycerol has a high viscosity because of the high capacity to form H bonds
Viscosity also increases as molecular complexity increases because the molecules become
“entangled” with themselves and one another.
Example:
Gasoline: non-viscous
Formula: CH
3
– (CH
2
) n
– CH
3
where n is from 3-8
Grease: very viscous
Formula: CH
3
– (CH
2
) n
– CH
3
where n is from 20-25
SOLIDS:
Two types:
1.
Amorphous solids: disordered structure
Example:
Glass – the components are “frozen” in place before it can achieve an ordered arrangement
2.
Crystalline solids: regular arrangement (in a crystal) lattice: 3D system of points designating position of components (atoms, ions or molecules) that make up the substance unit cell: smallest repeating unit of a lattice
3 common crystals:
Simple cubic (SC) 1 atom per unit cell
Body centered cubic (BCC) 4 atoms per unit cell
Face centered cubic (FCC) 8 atoms per unit cell
The extended structures shown below are a series of repeating unit cells that share common forces in the interior of a solid.
10_213 Unit cell Lattice Example
(a)
Polonium metal
Simple cubic
(b)
Uranium metal
Body-centered
cubic
(c)
Gold metal
Face-centered
cubic
Simple Cubic:
1 atom per unit cell
side length (d
0
= 2r)
Body Centered Cubic:
2 atoms per cell
body diagonal = d
0 √3
= 4r
d o √2
= diagonal through the base of cube.
Face Centered Cubic:
4 atoms per cell
Face diagonal = d
0 √2
= 4r
d
0 √2
= diagonal through face of the cube
10_221
(a) (b) (c)
1
8 atom
1
2 atom
Using Bragg’s equation:
X-ray diffraction: beams of light scattered from a regular array of points in which the spacings between components are comparable with the wavelength of light.
Constructive interference – in phase
Destructive interference – out of phase
Label the following waves as constructive or destructive
10_214
Waves in phase before striking atoms d 1 d
2
Waves still in phase
Waves reinforce each other, since
( d
2
- d
1
) is an integral number of
X- ray wavelengths.
Waves in phase before striking atoms d 1 d
2
No resultant wave
Waves cancel, because in this case
( d
2
- d
1
) is one half
X- ray wavelengths.
Bragg’s equation:
Used for analysis of crystal structures and to calculate the distance between planes in crystals.
The distance traveled by the x-rays is an integral number of wavelengths n
λ
= 2d sin
θ
d = distance between atoms n = an integer
λ = wavelength of the x-rays
θ = angle of reflection
Can you think of a practical application of Bragg’s equation?
Example problem: (make sure your calculator is in degrees!)
X rays of wavelength 1.54 Å were used to analyze an aluminum crystal. A reflection was produced at θ = 19.3 degrees. Assuming n = 1, calculate the distance, d, between the planes of atoms producing this reflection.
3 types of Crystalline Solids: classified according to what type of component occupies the lattice points. (see table bleow)
Classification of Solids:
Components that occupy lattice points
Bonding
Metallic metal atoms
Atomic Solids
Network non-metal atoms
Group 13 group 13 atoms
Molecular
Solids discrete molecules
Ionic
Solids ions
Ionic
Properties
Examples delocalized covalent range of hardness and melting points, conductor silver, iron, brass directional covalent
(molecules) hard, high melting point, insulator diamond
London
Dispersion forces very low melting poins solid argon is another example
Dipole-Dipole and/or
L. D. F. low melting points, insulator ice, dry ice high melting points
NaCl, CaF
2
What is the difference between ionic solids and molecular solids?
10_216
Label the diagrams to the left as:
Ionic solid
Atomic solid
Molecular solid
(a)
= C
Diamond
(b)
= Cl
= Na
Sodium chloride
(c)
Ice
= H
2
O
Packing in Metals:
Model: Packing uniform, hard spheres to best use available space. This is called closest packing.
Each atom has 12 nearest neighbors. (pictures on next pages)
-
hexagonal closest packed (“aba”)
-
cubic closest packed (“abc”)
1.
Closest packing arrangement of uniform spheres -- aba. This forms hexagonal closest packed -- hcp.
2.
Atoms arranged in aba pattern forming hexagonal closest packed (hcp) structure -- 2 atoms/cell.
10_217
(a)
View from above
(b)
View from side
(c)
Hexagonal closest packed structure – central atom has 12 nearest neighbors.
10_220 b
9
7
8 a
6
5
1
4
2
3 b
10
11
12 hcp
Face-centered cubic is cubic closest packed (ccp). The spheres are packed in an abc arrangement.
Calculate density of a closest packed solid
Silver crystallizes in a cubic closest packed structure. The radius of a silver atom is 144 pm.
Calculate the density of solid silver.
Bonding Models for Metals:
Electron Sea Model: A regular array of metals in a “sea” of electrons.
Band (Molecular Orbital) Model: Electrons assumed to travel around metal crystal in MOs formed from valence atomic orbitals of metal atoms.
Conduction Bands: closely spaced empty molecular orbitals allow conductivity of heat and electricity.
Representation of the energy levels (bands) in a magnesium crystal. 1s, 2s, & 2p orbitals are localized, but 3s & 3p orbitals are delocalized to make molecular orbitals.
10_225
3 p
3 s
Empty MOs
Filled MOs
2 p
2 s
1 s
12+ 12+ 12+ 12+ 12+
Magnesium atoms
Metal Alloys:
Substances that have a mixture of elements and metallic properties.
1.
Substitutional Alloy: some metal atoms replaced by others of similar size.
( brass = Cu/Zn)
2.
Interstitial Alloy: Interstices (holes) in closest packed metal structure are occupied by small atoms.
(steel = iron + carbon)
3.
Both types: Alloy steels contain a mix of substitutional (Cr, Mo) and interstitial (Carbon) alloys
Network Solids: Composed of strong directional covalent bonds that are best viewed as a “giant molecule”
brittle
do not conduct heat or electricity
carbon, silicon-based
Examples: graphite, diamond, ceramics, glass
10_229
(a) Diamond
Semiconductors: A substance in which some electrons can cross the band gap
-
Conductivity is enhanced by doping with group 3a or group 5a elements. (13, 15)
n-type semiconductor -- doped with atoms having more valence electrons -- Phosphorus.
p-type semiconductor -- doped with atoms having fewer valence electrons -- Boron.
-
See Figure 10.31 on page 478 in Zumdahl.
Molecular Solids:
• molecular units at each lattice position.
• strong covalent bonding within molecules.
• relatively weak forces between molecules.
• London Dispersion Forces -- CO
2
, I
2
, P
4
, & S
8
.
• Hydrogen Bonding -- H
2
O, NH
3
, & HF.
Trigonal, Tetrahedral and Octahedral Holes:
1.
Trigonal holes -- formed by three spheres in the same layer.
2.
Tetrahedral holes -- formed when a sphere sits in the dimple of three spheres in an adjacent layer.
3.
Octahedral holes -- formed between two sets of spheres in adjoining layers of closest packed structures.
10_238
Trigonal hole
(a)
Tetrahedral hole
(b)
Octahedral hole
(c)
Heagonal or Cubic closest packed:
octahedral hole for each atom or ion.
-
2 tetrahedral holes for each atom or ion.
simple cubic and body-centered cubic are not closest packed structures!
10_239
(a) (b) (c)
ZnS
(Above)The location (x) of a tetrahedral hole in the face-entered cubic unit cell. The S 2 ions are closest packed with the Zn 2+ ions in alternating tetrahedral holes.
10_240
(a)
(b)
(Above)The location (x) of an octahedral hole in the face-centered cubic unit cell. The Cl ions have a ccp arrangement with the Na + ions in all the octahedral holes.
Vapor Pressure: is the pressure of the vapor present at equilibrium.
. . . is determined principally by the size of the intermolecular forces in the liquid.
. . . increases significantly with temperature.
Volatile liquids have high vapor pressures.
Low boiling point
• _____ vapor pressure.
• _____ intermolecular forces.
Low vapor pressure
• _____ molar masses.
• _____ intermolecular forces.
Boltzman Distribution (below)-- number of molecules in a liquid with a given energy versus kinetic energy at two different temperatures.
10_245
(a)
T
1
Energy needed to overcome intermolecular forces in liquid
Kinetic energy (b)
Energy needed to overcome intermolecular forces in liquid
T
2
Kinetic energy
Natural Log of Vapor Pressure Versus Reciprocal Kelvin Temperature: y = mx + b ln (P vap
)
H vap
R
C
Slope = If the slope is known, then H can be calculated.
Clausius-Clayperon Equation: ln
P
P
2
1
Temperatures must be expressed in Kelvin.
H 1
1
R T
1
T
2
Example 10.6 on page 487 in Zumdahl.
The vapor pressure of water at 25 ˚ C is 23.8 torr, and the heat of vaporization of water at 25 ˚ C is
43.9 kJ/mol. Calculate the vapor pressure of water at 50.
˚ C
(use Clausius-Clayperon Equation to solve)
Sublimation:
• Change of a solid directly to a vapor without passing through the liquid state.
• Iodine
• Dry Ice
• Moth Balls
Melting Point: Molecules break loose from lattice points and solid changes to liquid. (Temperature is constant as melting occurs.) vapor pressure of solid = vapor pressure of liquid
Boiling Point: Constant temperature when added energy is used to vaporize the liquid. vapor pressure of liquid = pressure of surrounding atmosphere
Heating curve for water.
H = (m Ts) ice
+ m H f
+ (m Ts) water
+ m H v
+ (m Ts) steam
10_247
140
120
100
80
60
40
20
0
– 20
Ice and water
Ice
Water
Steam
Water and steam
Time
10_249
Water vapor
Solid Liquid water water
Solid and liquid water interact only through the vapor state.
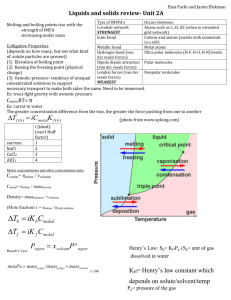
Phase Diagram:
Represents phases as a function of temperature and pressure.
critical temperature: temperature above which the vapor cannot be liquefied.
critical pressure: pressure required to liquefy AT the critical temperature.
critical point: critical temperature and pressure (for water, T c
= 374°C and 218 atm).
Phase diagram for water -- T m
is the regular melting point. The solid/liquid line has a negative slope.
10_252
Critical point
P c
= 218
Solid Liquid
1.00
P
3
= 0.0060
Triple point
Gas
T m
0
T
3
0.0098
T b
T c
100 374
Temperature ( ° C)
Phase diagram for carbon dioxide -- the solid/liquid line has a positive slope.
10_255
Critical point
P c
=
72.8
Liquid
Solid
P
3
=
5.1
Gas
Triple point
1.00
T m
T
3
– 78 – 56.6
T c
31
Temperature (°C)
Note the different scales why does carbon dioxide sublimate at STP?
Phase diagram for sulfur -- note the two different solid forms of rhombic and monoclinic sulfur.
10_256
Liquid
Rhombic
Monoclinic
(119°C, 0.0027 mm Hg)
(96°C, 0.0043 mmHg)
Vapor
Temperature (°C)
Phase diagram for carbon -- note the two solid forms of diamond and graphite.
10_257
10 11
Diamond
Liquid
10 9
Graphite
10 7
Vapor
0 2000 4000
Temperature (K)
6000