CH 5. a. Students know the observable properties of acids, bases
advertisement

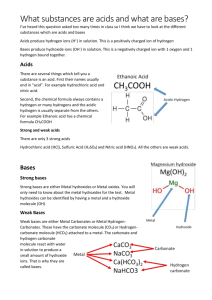
ICS1 Chemistry 5 Students who learn the concepts in this standard set will be able to understand and explain aqueous acid–base reactions, properties of acids and bases, and pH as a measure of acidity and basicity. Careful thought should be given to how this standard set best fits with the other chemistry standards. It may be desirable to cover Standard Set 6, “Solutions,” in this section first so that students will know about the aqueous dissolving process and about concentration calculations and units. Familiarity with Standard Set 9, “Chemical Equilibrium,” in this section may help students to conceptualize the strengths of acids and bases. Students should be able to represent and balance chemical reactions. They should also be able to interpret periodic trends in electronegativity for the upper two rows of the periodic table (see Standard Set 1, “Atomic and Molecular Structure,” in this section). Hydrogen with its low electronegativity easily + − forms the positive hydrogen ion, H . Students need to know the charge and formula of the hydroxide ion, OH . With knowledge of polar covalent bonding, students should be able to distinguish between two important types of neutral molecular compounds: those that dissolve in an aqueous solution and remain almost completely as neutral molecules and those that dissolve in an aqueous solution and partially or almost completely produce charged ions (see Standard Sets 1 and 2 in this section). Students should be able to compare the three descriptions of acids and bases— the Arrhenius, Brønsted-Lowry, and Lewis acid–base definitions—and recognize electron lone pairs on Lewis dot structures of molecules (see Standard Set 2, “Chemical Bonds,” in this section). To calculate pH, students should understand and be able to use base-10 logarithms and antilogarithms and know how to obtain logarithms by using a calculator. Students should + − become proficient at converting between pH, pOH, [H ], and [OH ]. CH 5. Acids, bases, and salts are three classes of compounds that form ions in water solutions. As a basis for understanding this concept: Standard CH 5. a. Students know the observable properties of acids, bases, and salt solutions. Framework Comparing and contrasting the properties of acids and bases provide a context for understanding their behavior. Some observable properties of acids are that they taste sour; change the color of litmus paper from blue to red; indicate acidic values on universal indicator paper; react with certain metals to produce hydrogen gas; and react with metal hydroxides, or bases, to produce water and a salt. Some observable properties of bases are that basic substances taste bitter or feel slippery; change the color of litmus paper from red to blue; indicate basic values on universal indicator paper; and react with many compounds containing hydrogen ions, or acids, to produce water and a salt. These properties can be effectively demonstrated by using extracted pigment from red cabbage as an indicator to analyze solutions of household ammonia and white vinegar at various concentrations. When the indicator is added, basic solutions turn green, and acidic solutions turn red. Students can also use universal indicator solutions to test common household substances. Students need to follow established safety procedures while conducting experiments. Standard CH 5. c. Students know strong acids and bases fully dissociate and weak acids and bases partially dissociate. Framework Acids dissociate by donating hydrogen ions, and bases ionize by dissociating to form hydroxide ions (from a hydroxide salt) or by accepting hydrogen ions. Some acids and bases either dissociate or ionize almost completely, and others do so only partially. Nearly complete dissociation is strong; partial dissociation is weak. The strength of an acid or a base can vary, depending on such conditions as temperature and concentration. CH 5. Acids, bases, and salts are three classes of compounds that form ions in water solutions. Standard CH 5. a. Students know the observable properties of acids, bases, and salt solutions. Standard CH 5. c. Students know strong acids and bases fully dissociate and weak acids and bases partially dissociate. Draw picture of an Acid and Base Make sure to color by Friday Framework Ch5.a properties of acids: sour; color of litmus paper blue to red; acidic values on universal paper; react w/ metals to produce hydrogen gas; react w/ metal hydroxides/bases, produce water and a salt. properties of bases: bitter or slippery; litmus paper red to blue; basic values on universal paper; react w/ compounds of hydrogen ions /acids, produce water and a salt. Framework CH 5.c Arrhenius acid: generates [H+] in solution base: generates [OH-] in solution eqn: acid + base salt + water ex: HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) Brønsted-Lowery: acid: donates a [H+] (proton donor) base: accepts a [H+] (proton acceptor) eqn: acid + base acid + base ex: HNO2(aq) + H2O(aq) NO2-(aq)+ + H3O (aq) Lewis: acid: accepts an electron pair base: donates an electron pair The advantage of this theory is that many more reactions can be considered acid-base reactions because they do not have to occur in solution. Salts is formed when an acid and a base are mixed and the acid releases H+ ions while the base releases OH- ions. This process is called hydrolysis. The pH of the salt depends on the strengths of the original acids and bases: Acid Base strong strong pH = 7 Salt pH weak strong pH > 7 strong weak pH < 7 weak weak depends on which is stronger Heading Wk 7 Ch 5a 5c Acid and Base Write questions 1-5 Leave room under each question (“like 3 lines”) “Like usual due Friday” test friday 1. Equal volumes of 1 molar hydrochloric acid (HCl) and 1 molar sodium hydroxide base (NaOH) are mixed. After mixing, the solution will be… A strongly acidic B weakly acidic C nearly neutral D weakly basic 2. The above picture shows a light bulb connected to a battery with the circuit interrupted by a solution. When dissolved in the water to form a 1.0 molar solution, all of the following substances will complete a circuit allowing the bulb to light except A hydrochloric acid. B sodium nitrate. C sucrose. D ammonium sulfate. 3. Which of the following is an observable property of many acids? A They become slippery when reacting with water. B They react with metals to release hydrogen gas. C They produce salts when mixed with other acids. D They become more acidic when mixed with a base. 4. Potassium hydroxide (KOH) is a strong base because it A easily releases hydroxide ions. B does not dissolve in water. C reacts to form salt crystals in water. D does not conduct an electric current. 5. Of four different laboratory solutions, the solution with the highest acidity has a pH of A 11.B 7.C 5.D 3.