A comparison on the performance of zinc oxide and hematite
advertisement

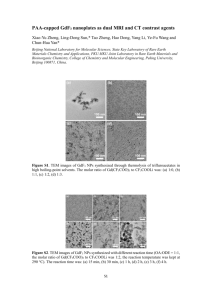
A comparison on the performance of zinc oxide and hematite nanoparticles for sensitive and selective detection of paranitrophenol Kulvinder Singh,a Appanjeet Kaur,a Ahmad Umar,b G. R. Chaudhary,a Sukhjinder Singh,a and S. K. Mehta*a a Department of Chemistry and Centre of Advanced Studies in Chemistry, Panjab University, Chandigarh 160 014, India b Promising Centre for Sensors and Electronic Devices (PCSED), Centre for Advanced Materials and Nano-Research (CAMNR), Najran University, P.O. Box 1988, Najran, 11001, Kingdom of Saudi Arabia * Authors to whom correspondence should be addressed; E. mail: skmehta@pu.ac.in (S.K.Mehta) Tel.: +91-172 2534423 Fax: +91-172-2545074 1 Supplementary Material Characterization of ZnO NPs Figure S1 (a) TEM image of ZnO NPs (b) XRD pattern of ZnO NPs Figure S1 (a) depicts the TEM image of as synthesized ZnO particles. This shows that the particles are spherical in nature with the diameter of around 30±5 nm. To confirm the crystallinity of the particles, X-ray Diffraction (XRD) pattern has been studied (Fig. S1(b)). The well intense peaks show that the NPs are well crystalline in nature. The entire XRD spectrum matches with wurtzite phase of ZnO NPs and exhibit full reliability with JCPDS card no. 36-1451[1]. The crystalline size using Debye-Scherrer formula (eq. 1) comes out to be 24.85 nm that is consistent with the TEM image size. 𝑑 = 0.94𝜆⁄𝛽𝑐𝑜𝑠𝜃 (1) Where d is the size of material, λ is the wavelength of X-ray, β is the full length at half maximum of the peak in radian and θ is the angle of diffraction peak. Characterization of α-Fe2O3 NPs Figure S2 (a) TEM image of α-Fe2O3 NPs (b) XRD pattern of α-Fe2O3 NPs 2 Figure S2 (a) depicts the typical TEM image of as synthesized α-Fe2O3 NPs. The size of the NPs ranges from 60-80 nm. Figure S2 (b) depicts the XRD spectrum of α-Fe2O3 NPs. All the diffraction peaks matched well with the XRD spectrum of α-Fe2O3 NPs reported in literature which is in accordance with the rhombohedral α-Fe2O3 structure as mentioned in the JCPDS card number 84–0311 [2]. The crystalline size of α-Fe2O3 NPs using eq. 1 comes out to be 38.05 nm. The peak at 21.36 arises due (110) plane α-FeOOH phase that is formed due to initial conversion of iron hydroxide to α-Fe2O3 NPs [3]. Figure S3 Electrochemical response of bare Au electrode with PNP. Figure S3 depicts the electrochemical response of bare Au electrode with PNP. It can be seen that with bare electrode we were not able to get a redox couple, instead only one reduction peak is obtained at -0.26 V. Also the response of Au electrode is not to good for the reduction peak as compare to the modified electrode. The morphology of both the NPs has been examined with the help of FESEM. Figure S4 depicts the typical FESEM images of ZnO and α-Fe2O3 NPs. Figure S4 FESEM images of (a) ZnO (b) α-Fe2O3 NPs 3 [1] Wahab R, Kim Y S, Lee D S, Seo J M, Shin H S (2010) Controlled Synthesis of Zinc Oxide Nanoneedles and Their Transformation to Microflowers. Sci Adv Mater 2:35-42. [2] Umar A, Abaker M, Faisal M, Hwang S W, Baskoutas S, Al-Sayari S A (2011) High-yield synthesis of well-crystalline α-Fe2O3 nanoparticles: structural, optical and photocatalytic properties. J Nanosci Nanotechnol 11:3474-3480. [3] Hua M, Jian L, YueQiang L, XiaoDong L, QingMei Z, Jun F (2011) Characterization of γ-Fe2O3 nanoparticles prepared by transformation of α-FeOOH Chinese Sci Bull 56:2383-2388. 4