File
advertisement

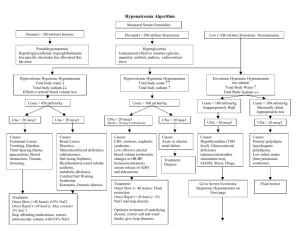
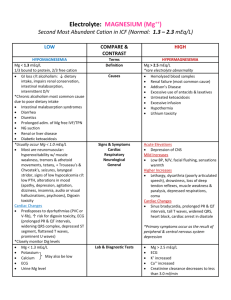
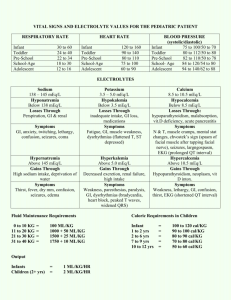
Nayri Hatsakorzian Pharm.D/MPH candidate 2014 Endogenous fluids and electrolytes 1- Plasma a. 55% of whole blood b. Solution: H2O (90%) + electrolytes + proteins + waste products i. Major cations: (Na, K, Ca, Mg) ii. Minor cations: Fe, Cu, Zn, H iii. Major anions: Cl, HCO3 iv. Minor anions: Phosphate, lactate, sulfate v. Major ptn: ALB, globulins, clotting factors vi. Waste products include: bilirubin, BUN, creatinine, uric acid 2- Cellular a. RBC: 45% of the whole blood volume b. Platelets and WBC 3- Functions a. Circulates nutrients, waste product b. Circulates immune and endocrine system products c. Medium for pH and thermal regulation Different types of pressures - Oncotic pressure (colloid osmotic pressure): depends on the protein concentration and the reflection coefficient of the capillary wall. Albumin generates about 70% of the oncotic pressure. Oncotic pressure is usually responsible of shifting fluids into plasma. - Hydrostatic pressure: pressure exerted by the fluid. Imbalance drives fluids out of the capillaries. Na and H2O Homeostasis - Balance between intake vs. output; this controls volume regulation and osmoregulation - H2O and /or Na is maintained through sensible (ingestion) and insensible (metabolic H2O production) intake and sensible (urinary + GI tract) and insensible (skin + respiratory tract) output. - Na is the primary extracellular cation, maintain osmolality (# of particles/kg of H2O), and is a dissociable solute (1 mmol of NaCl= 1 mmol of Na and 1 mmol of Cl = 2 mOsm) - An increase in volume increase in BP atrial distension atrial natriuretic peptide 1- Increase in Na and H2O excretion through kidney decrease in renin secretion decrease in aldosterone secretion through the adrenal cortex peripheral vascular dilation and decrease in catecholamine secretion decrease or maintaining normal BP 2- also, increase in BP due to increase in volume increase stimulation of carotid sinus and carotid arch baroreceptors decrease in sympathetic output vasodilation Hyponatremia - Increase in ECF volume, decrease in free H2O elimination (or sensible intake > elimination), and/or increase in AVP secretion (causes vasoconstriction and prevent water elimination) are major causative factors for hyponatremia. - There are three types of hyponatremia: o Hyperosmotic: hyprglycemia, administration of glycerin or mannitol solution exogenously, causing increase in osmotic pressure which in turn causes intracellular fluid to shift extracellularly leading to increase in volume and decrease sodium concentration o Isoosmotic: increase in osmotically active substances in intravascular space o Hypoosmotic: which is also divided into three types: Hypovolemic: (true sodium deptetion): V/D, diuretics, fistula losses, burns, hypoaldosteronism (can lead to sodium excretion) Isovolemic: SIADH (results in water retension), psychogenic polydipsia (hypotonic solution intake) Hypervolemic: CHF, cirrhosis, hypoalbuminemia, nephrotic syndrome Presentation of hyponatremia: - Mild hyponatremia: asymptomatic to decrease in mental status, posture, gait, increase risk of fall. - Severe hyponatremia: decrease in cellular function + increase in intracranial pressure leading to decrease in CNS perfusion and function. In most cases, magnitude and rate of sodium reduction determine severity of symptoms and cellular complications. Treatment of hyponatremia: - Manage underlying condition - Rapid correction of hyponatremia is not recommended; risks vs benefit assessment is required - Max safe rate to increase sodium is 0.5 mEq/L/Hr or 10 mEq/day - NS (154 mEq/L) o Effectively corrects Na and H2O deficit o Provides volume resuscitation o Most appropriate for hypovolemic patients - 3% NaCl (513 mEq/L) o Reserved for severe Na (<115) and patients requiring minimum volume replacement o Provides rapid reversal o Max infusion rate is 136ml/hr in a 70kg patient - Misc tx o Restrict fluids (use minimum amount of fluids when administering ABX) o Sodium tablets o Furosemide in case of hypervolemic hyponatremia o Demeclocyline: decrease AVP production, contraindicated in patients with liver diseases o Conivaptan: V2 receptor antagonist leading to decrease in AVP on kidneys; avoid in patients with hypovolemia o Fluid and Na restriction as long term therapy Hypernatremia - Less common than hyponatremia; usually due to dehydration due to blunted thirst response - Three types of hypernatremia: o Hypovolemic: due to intake of high sodium containing fluids with free H2O loss o Isovolemic: diabetic insipidus decrease in AVP o Hypervolemic: increase intake of high sodium fluids; NS or 3% saline fluid resuscitation Presentation of hypernatremia: - Dry mucosa, hypotension, tachycardia, weakness, lethargy, confusion, restlessness, muscle twitching, hyperreflexia, seizures, coma, and death Treatment of hypernatremia: - Manage underlying condition - Max sodium reduction is 0.5mEq/L/hr or 10 mEq/day; close monitoring of sodium is required every 2-4 hours - D5W o Provides free H2O - NS - o Provides IVF resuscitation; with no intracellular shift D5W-1/2 NS o Provides resuscitation of ICF & ECF If hypernatremia is due to hyperglycemia induced osmotic diuresis insulin + NS Potassium - Determines the ICF, since it is mainly intracellular cation - Necessary for establishing resting potential across cell membrane to regulate nerve and muscular function, enzymatic reactions, protein and glycogen synthesis, cardiac conduction and function. K is primarily regulated by the kidneys through filtration, reabsorption and excretion. Serum levels can be affected by renal function, serum pH, insulin/glucagon imbalance, and drugs. In general, a small intravascular reduction can be a sign of massive intracellular deficit. - Aldosterone plays an important role in K homeostasis increase in K causes increase in aldosterone secretion K elimination by kidneys - Insulin causes Na/K ATPase activity causing an extracellular to intracellular shift - Epinephrine (catecholamines) also cause increase in Na/K ATPase activity and intracellular shift - Acidosis: a decrease in pH= increase in H ions shifts intracellular K increase plasma K - Alkalosis: an increase in pH= decrease in H ions shifts extracellular K decrease in plasma K Hypokalemia Occur through GI losses, medication uses (loop diuretics, cisplatin) Presentation of hypokalemia: - Generally non specific and variable; mild hypokalemia (3-3.5) is usually asymptomatic - Moderate (2.5-3) cramping, weakness, malaise, myalgia - Severe (<2.5) cardiac dysfunction, arrhythmias Treatment of hypokalemia: - Goal is to prevent life threatening complications, but avoid over-correction - Bolus of K at 10mEq/hr - Close monitoring with EKG Hyperkalemia A much more dangerous phenomenon than hypokalemia. Usually due to kidney failure decrease in K elimination accumulation. K sparing diuretics (aldosterone antagonists), dietary substitution, cellular lysis, and acidosis are the most common etiologies. Severity ranges from mild (5.5-6), moderate (6.1-6.9), to severe > 7 mEq/L Treatment of hyperkalemia: - There are three approaches to hyperkalemia 1- Antagonizing the membrane effects of K with Ca. 2- Driving extracellular K back into cells 3- Removing excess K from body - 1st line therapy is calcium gluconate, preferably infused into a central or deep vein d/t its vesicant characteristic and possibility of tissue necrosis upon extravasation, along with insulin-glucose duo. Calcium will directly antagonize the membrane action of hyperkalemia. Calcium gluconate IV starts showing its effects within minutes, but it is short lived. Ca should not be administered as monotherapy and it should be combined with agents that drive K shifting. In almost all patients potassium concentration drops by 0.5 to 1.2 mEq/L. - Albuterol is considered transient therapy in patients who have symptoms or serious ECG manifestations despite Ca and insulin with glucose therapy. Albuterol along with insulin and glucose - has additive effects and reduce k concentration by approximately 1.2 to 1.5 mEq/L. albuterol is widely used in ESRD patients. Sodium bicarbonate (50-100 mEq IV over 2-5 min) can be used to raise the systemic pH, which results in hydrogen ion release from cells as part of the buffering reaction. This change is accompanied by K movement into the cells to maintain electroneutrality. Loop or thiazide diuretics, which works by increasing K loss in urine. Cation exchange resin with sodium polystyrene sulfonate (SPS); this treatment is not preferred d/t intestinal necrosis. Dialysis: if patient has dialysis port, it is preferable to go through dialysis rather than administering SPS. Calcium Almost exclusively found in skeletal bone. It maintains cell membrane integrity, neuromuscular activity, bone homeostasis, as well as regulating endocrine/exocrine functions. It is regulated by parathyroid hormone, calcitonin, serum phosphate, and Vit D. Ca levels should be corrected to account for bind and unbound Ca in the body, which has direct correlation with albumin. Cacorr= Cauncorr+(0.8 * (albumin-4)) Hypercalcemia There are different causes of hypercalcemia - malignancies (esp. bone CA or metastasis) - hyperparathyroidism: causes release of Ca from bones - exogenous drugs: Ca supplements, thiazide diuretics Various signs and symptoms: - GI (N/V, constipation, anorexia, abdominal pain) - Neuromuscular (decrease reflexes, weakness, lethargy) - Cardiovascular (abnormal EKG “shortened QT”) - Neurologic (obtundation, mental status changes, coma) - Renal (polyuria, polydipsia, nocturia, nephrolithiasis, CRI) Treatment: - Isotonic saline hydration: increase urinary Ca excretion and restores intravascular volume - Calcitonin: inhibits bone resorption via osteoclast inhibition, along with Ca excretion - Bisphosphonates: inhibits bone resorption via osteoclast function and recruitment inhibition - Loop diuretics: increases excretion and prevent reabsorption in the loop of Henle - Calcimimetics: Ca sensing receptor agonist, reduces PTH (parathyroid carcinoma, secondary hyperparathyroidism in CKD). Agents include: Cinacalcet Hypocalcemia Mainly caused by vitamin D deficiency, exogenous drugs, hypoparathyroidism, hyperphosphotemia, and hypomagnesemia. Signs and symptoms include: - Neuromuscular: finger numbness, paresthesis of lips and extremities, neuropathies of extremities, fatigue, and cramps - Cardiovascular: arrhythmias, myocardial failure, hypotension - Neurologic: Depression, confusion, hallucination, memory loss, seizures - Dermatologic: brittle grooved nails, dry skin Magnesium Found in bone and intracellular fluid. An important cation in modulating neuromuscular activity and enzymatic system maintenance. Mainly regulated by serum calcium, phosphate serum concentration, and renal function. Hypermagnesemia Results from decreased excretion in renal failure and/or increase intake (laxative abuse). Sings and symptoms include: - General: N/V, sweating, flushing - Neuromuscular: decreased in reflexes, weakness, flaccid paralysis, fatigue, respiratory depression - Neurologic: lethargy, confusion, sedation - Cardiovascular: bradycardia, hypotension, arrhythmias, heart block, asystole, cardiac arrest Stopping the offending agent should be sufficient to treat hypermagnesemia with normal renal function patients. If renal function is compromised then loop diuretics is consider along with dialysis if GFR < 15 Hypomagnesemia Seen in malnourished patients, hospitalized patient due to NG suctioning and overuse of diuretics. Signs and symptoms include: - Neuromuscular: tremors, weakness, muscle cramps, tetany, nystagmus - Neurologic: disorientation, psychosis, seizures, coma - Cardiovascular: EKG changes, arrhythmias, hypotension - Electrolyte imbalance: refractory hypokalemia and hypocalcemia Treatment: - 1 to 2 grams of magnesium sulfate (8 to 16 meq [4 to 8 mmol]) in 50 to 100 mL of D5W or NS can be given initially over 5 to 60 minutes followed by an infusion - Infusion regimen can be 4 to 8 g magnesium sulfate (32 to 64 meq [16 to 32 mmol]) given slowly over 12 to 24 hours. This dose can be repeated as necessary to maintain the plasma magnesium concentration above 1.0 mg/dL. - Patients with renal insufficiency (CrCl < 30 mL/min) are at risk for severe hypermagnesemia if large doses of magnesium are given. Reduce by 50% with close magnesium serum concentration is warranted in renal insufficiency patients. - Oral agents include: Magnesium oxide 400mg daily, OTC Mg chloride Phosphate Found mostly in bones and ICF. Maintains and makes up cell membranes, enzymes and is involved in energy transfer, acid-base buffering, muscle contraction, regulating carb/protein/fat metabolism, and neurologic functions. It is regulated by Ca, Al concentrations, vit D, parathyroid hormone, renal glomerular filtration, loop and tubular reabsorption, and small intestinal absorption. Hyperphosphatemia Can be due to decrease excretion, transcellular shift, and increased intake. It mostly asymptomatic, but it can result in muscle cramps as well. Hypophosphatemia Can be due to - Increase excretion: N/V, diuresis - Transcellular shift - Decreased intake: starvation, malnutrition, malabsorption, phosphate binders - Increase requirement: refeeding syndrome - Drugs: calcitonin, glucagon, beta agonists Signs and symptoms include: - Neurologic: irritability, encephalopathy, confusion, obtundation, seizures, coma - Neuromuscular: Numbness, weakness, parasthesias, respiratory failure - Cardiovascular: platelet dysfunction, decreased cardiac contractility, and cardiac failure References: Dipiro et al. Concepts in Clinical Pharmacokinetics. 2005. 4th Ed. UpToDate. Inc., Lexicomp. Inc.,