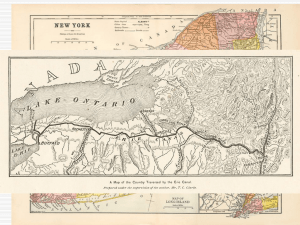
except
advertisement

Chem 1105 Exam 2 25 November 2008 75 points Name_____________________________ I. Multiple Choice (12 @ 3 points = 36 points) 1. One of the first uses of the steam engine was to a. Power trains b. **Pump water out of mines c. Weave cloth d. Smelt iron e. Heat homes 2. Sea salt was used in all of these ways except to a. Preserve food and animal skins b. Serve as starting material for making soda ash c. Season food d. Serve as a starting material for bleach e. **Serve as a starting material for organic dyes 3. All of these aided the growth and development of the textile industry except a. Advances in energy sources. b. Issuance of patents. c. **Financial incentives for starting textile cottage industries. d. Colonization which provided natural resources and markets. e. Improvements in transportation. 1 4. The properties of the final pottery product depend on all of the following except a. Firing temperature. b. Presence or absence of glaze. c. Chemical composition of the clay components. d. Relative proportions of the clay components. e. **All of these contribute to defining pottery properties. 5. The early steam engines had all of these components except a. **A separate condenser b. Source of heat c. Expansion chamber d. Working fluid e. Compression chamber 6. All of these are true about potash except a. Potash was originally made by burning wood. b. Potash is also called an alkali. c. Barilla was imported from Spain as a source of potash. d. **Potash was first synthesized using sea salt, vitriol, limestone and charcoal. e. Kelp was short-lived but important source of potash. 2 7. The first organic dye successfully synthesized was a. Indigo b. Aniline c. **Mauve d. Barilla e. Quinine 8. Pottery glaze was applied for all of these reasons except to a. Strengthen pottery b. Decorate product c. **Simplify the “throwing” or shaping of the product d. Make the pottery less porous e. Add color 9. The efficiency of a steam engine is governed by a. The First Law of Thermodynamics b. The Law of Conservation of Mass c. The Laws of Gravity d. The Law of Electrostatic Forces e. **The Second Law of Thermodynamics 3 10. According to the material in Clow, the Ninth Earl of Dundonald was involved in all of the following industries except a. **Vitriol b. Sea salt c. Coal Tar d. Soda Ash e. Soap 11. Bleach removes stains and dirt by all of the following methods except a. Oxidize stains by adding oxygen. b. Convert double bonds to single bonds. c. Break bonds in the stain to decompose stain molecules. d. **Convert stain to matching dye color of fabric. e. Dissolves readily in water to help bleaching action. 12. The two most abundant elements in pottery clay are a. Iron and aluminum b. **Silicon and oxygen c. Chlorine and sodium d. Carbon and hydrogen e. Hydrogen and oxygen 4 II. Short essays (Write in the Blue Exam Book) 1. (20 points) Pick two of these to write about; clarity, factual material, specific examples and good writing will be rewarded. a. Describe, contrast and compare the three early steam engines we studied. The development and increasing utilization of the steam engine was one of the most important advances of the Industrial Revolution. We studied the Savery, Newcomen and Watt steam engines. All of these converted heat to work replacing human and animal effort and water power to run machines with steam power. Each had a 1) boiler to burn fuel and convert liquid to gaseous water which involved a very large expansion of the gas and a 2) condenser to liquefy the water vapour while undergoing a compressor. This expansion and compressor cycle was integrated with a mechanical device (e.g. arm) to pump water out of mines, turn a grain grinder, power a train, etc. The Savery engine had a series of valves which opened and closed to create a vacuum to move the mechanical device. Its power rating was about 1 hp but was susceptible to explosions. It was used to pump water out of mines and was replaced by the Newcomen engine. This engine, called the atmospheric engine, had a cylinder and piston design (i.e. it had a moving piston which responded to the expansion and compression of water). Its power rating was about 5 hp and increased the depth of the mines that could be pumped out. The innovation of the Watt engine is that it had a separate condenser which could be isolated from the main cylinder. This condenser was cooled so the entire cylinder could remain hot during the compression step. This saved 75% of the fuel costs; the Watt engine provided about 5-10 hp. Watt’s 25 year partnership with Boulton was a very lucrative one as Boulton had a manufactory at Soho House, capitol, skilled workers, an existing network of business associates and potential markets. 5 b. Describe, contrast and compare the three mechanisms for applying dye to fabric. The early materials used for fabric were natural products like cotton, flax and wool. The chemical in these materials is cellulose which is a carbohydrate with C-OH groups available for hydrogen bonding to the dye. If the dye also has C-OH groups, it formed strong H-bonds with cellulose (i.e. the dye bonded to the fabric). This is called direct dyeing. In some situations, a mordant or metal cation (Ca2+. Al3+, etc) was applied to cellulose to bond to the fabric as well as the dye. In vat dyeing, the dye cannot be applied using the previous two methods because it has no hydrophilic groups. Indigo is an example of a vat dye. Indigo is a deep purple dye extracted from a plant. The indigo molecule has two C = O groups which absorb light to go to an excited state and then emit visible purple light when it goes back to the ground state. The C = O group cannot hydrogen bond with the –OH groups on cellulose (fabric) so the indigo dye does not adhere to cellulose. When indigo is reduced, the C =O group are converted to C-OH groups which form H-bonds with cellulose, thus attaching to the fabric. The reduced leuco-indigo molecule is yellowish, but when the fabric dyed with it is exposed to air and light, the leuco-indigo is oxidized to purple indigo. c. Describe the modern chemical industry and compare it to the chemical industry developed during the Industrial Revolution. During the Industrial Revolution, the chemical industry referred to the production of chemical products from raw materials. The first chemicals were extracted from raw materials: potash from burning wood and later kelp soda ash from the same sources and later via a synthesis from salt, vitriol, limestone and carbon 6 chlorine from sea salt for bleach, soap making The products from coal tar were included on Exam 1. These were used in for use in the textile, glass and pottery industries. The modern chemical industry produces chemical products from raw materials and via synthesis of compounds. It is a $3 trillion enterprise worldwide and included petrochemicals, pharmaceuticals, inorganic compounds, agrichemicals. Most of the chemicals produced are using as starting materials for other industries; this is similar to the production of soda ash during the Industrial Revolution for the glass industry or the production of chlorine to bleach textiles. During the 18th and 21st centuries, hazardous waste, energy costs, raw material depletion and pollution were/are critical issues for the industry to address. d. Discuss the role of the Ninth Earl of Dundonald in the early chemical industry. The Ninth Earl of Dundonald was involved in the production of salt, potash, soda ash and soap. He wrote pamphlets on the production of salt, was concerned about the hazardous working conditions for women, lobbied against the salt tax and worked to develop new ways to purify salt. He was concerned about the production of potash and soda ash. He held a patent for producing soda ash, was involved in its synthesis and in the byproducts of this process. 2. (5 points) Based on your study of the steam engine, how does a refrigerator or air conditioner work? Be sure to identify heat in, heat out, work and efficiency in your answer. 7 The refrigerator and air conditioner are based on the steam engine cycle run in reverse. The goal is to provide energy or work to remove heat from a low temperature source (heat in, the refrigerator or room) and dump it to a high temperature sink (heat out, the kitchen or outside. The efficiency is the heat taken into the cycle from the refrigerator/ work provided. III. Matching (7 @ 2 points, 14 points) Match each compound or term with the correct formula (chemical or definition). a. Potash _10_____ b. Bleach _14_____ c. Efficiency of steam engine _6_____ d. Sea Salt _11_____ e. Soda Ash _7_____ f. Silica _3_____ g. Chromophore _1_____ 1. C = O 8. SiH4 2. CaCO3 9. C-C 3. SiO2 10. K2CO3 4. CuO 11. NaCl 5. H2SO4 12. C16H10N2O2 6. Work out/Heat in 13. Heat out/Heat in 7. Na2CO3 14. OCl- 8