answers to Atmosphere Revision Questions
advertisement

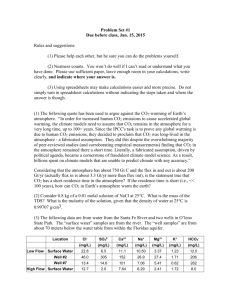
Atmosphere Revision Questions 1. List the 4 main gases present in tropospheric air with their % in the atmosphere Nitrogen 78 % Oxygen 21% Argon 1% Carbon dioxide 0.0036% 2. Helium is present at 5.24 ppm. What would this be in % composition. 5.24/1000000 x 100 = 0.000524 % or 5.24 x 10-4 3. List these in order of increasing frequency in the electromagnetic spectrum UV, red light,IR,blue light. IR, red, blue,UV 4. What types of radiation arrive from the sun at the earth’s surface and what frequencies are reflected back into the atmosphere.? UV from the sun, earth radiates lower frequency IR back 5. 6. Match the type of radiation to the energy gaps that they match i. Infrared Electronic ii. Microwave Vibrational iii. UV/ Visible Rotational 7. What does the phrase energy is quantised mean? In fixed amounts or energy levels. 8. Calculate the frequency of radiation which would cause a C – Br bond to photodissociate. The bond Enthalpy of C – Br is 290 kJmol -1 and Avogadros number L is 6.02 x 1023 .Plancks constant h is 6.63 x 10-34 JHz-1. (HINT first find the energy in Joules to break 1 C-Br bond.) 290 x 1000 / 6.02 x 1023 = 4.82 x 10-19 Joules to break 1 C-Br bond. Freq = E/h = 4.82 x 10-19 / 6.63 x 10-34 = 7.27 x 1014 Hz 9. Explain the difference between homolytic and heterolytic fission with reference to a C–Cl bond. Homolytic fission bond breaks and forms radicals usually light or heat 1 e goes each way Hetrolytic fission bond breaks to forms ions 2 es go to one atom 10. Define the activation energy of a reaction and explain why a low value of activation energy results in a high rate of reaction. Use a sketch to illustrate your answer. Minimum energy for a reaction to occur when molecules collide “fruitful collision” Low value more molecules have enough energy 11. Explain using collision theory why a small increase in temperature can cause a large in crease in the rate of reaction A small increase in temp means a large increase in no of molecules with Ea. 12. Define the term “homogeneous catalyst” and give an example explaining why an intermediate is important. Catalyst is in the same state as the reagent and speeds up the reaction by providing an alternative pathway with a lower value of Ea. 13. Sketch an enthalpy profile for an exothermic reaction with and without a catalyst. Label Reactants, Products, Enthalpy change ΔH, intermediate, activation energy Ea and Ea catalysed. e ΔH intermediate 14. What were CFC’s used for and what have they being replaced with? Aerosol propellants, refrigerants appropriate volatility and unreactive Replaced with HCFCs and HFC’s and hydrocarbons 15. Write equations to show the formation of ozone in the stratosphere and its breakdown. hv O2 2O O + O2 O3 O3 O + O2 16. Explain what happens to a halogenoalkane when it photodissociates . What effect do the halogen radicals formed have on Ozone levels. Light of a particular frequency causes homolytic fission of C-X and releases a halogen radical eg CH3Cl CH3 . + Cl . Cl then reacts with O3 in a catalytic cycle Cl + O3 ClO + O2 ClO + O O2 + Cl 17. Explain the term Dynamic equilibrium with reference to a half fill bottle of fizzy drink. Forward and reverse reaction occur at same rate concentrations remain constant CO2 gas escapes at the same rate as it dissolves in a closed system 18. Give an equation to show the equilibrium which occurs when CO2 (aq) reacts with water and explain why this is vital in removing CO2 from the atmosphere. CO2 (aq) + H2O(g) = HCO3- (aq) + H+ (aq) more CO2 in the atmosphere means more CO2 will dissolve increasing the concentration of CO2 (aq) due to Le Chateliers principle. Increased CO2 (aq) moves eqm to right so more HCO3- + H+ 19. Define a greenhouse gas using the term IR window explaining why this effect leads to a temperature increase and what evidence there is to show that rising CO2 levels are linked to Global Warming. Absorbs IR radiation as bonds vibrate more vigorously giving an increase in kinetic energy So temp rises. Temp increase matches a rise in CO2 levels. 20. Predict the effect of the following changes on the position of equilibrium for the exothermic reaction below a. b. c. d. e. CH4 (g) + H2O(g) CO(g) + 3 H2 (g) increasing the pressure favours reverse so left as 2g 4g increasing the temperature high T favours reverse endothermic so to the left adding a catalyst no change as forward and reverse both speed up equally adding more CH4 favours forward so to the right as more reactants removing the CO(g) favours forward so to the right as less products 21. Describe the structure of Silicon dioxide and carbon dioxide and explain the difference in states at RTP. A sketch would be useful SiO2 solid as v high T needed to break bonds CO2 gas giant covalent simple molecule weak ididis