Assignment-2-Absolute-vs-relative-age-dating
advertisement

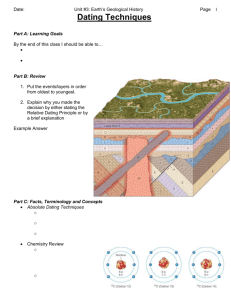
Name___________________________________________________________________________ Date__________________________________ Hour_________________ Assignment #2: Absolute vs. Relative Age Dating Directions: Read the introduction to answer the questions that follow. Then complete the step-by-step activity that follows. Part I Read the Introduction: Scientists use radioactive dating to find out the absolute age of an object or rock. They are able to do this because radioactive isotopes emit or let off nuclear particles at a regular rate during radioactive decay. This original radioactive isotope is called the parent. Over time as the parent isotope lets off particles it changes to another element called the daughter. Each isotope does this at a regular but different rate. By looking at the amount of parent isotope and daughter element in an object or rock the age can be calculated. During radioactive decay, radioactive isotopes break down into stable isotopes of other elements. These radioactive isotopes have half-lifes: this is the amount of time it takes for one-half of the original amount to decay. Scientists can also use relative-age dating to gain a clearer understanding of geologic events in Earth’s history. It does not allow scientists to determine exactly how many years ago an event occurs, but it can be somewhat helpful when studying geologic time. Relative-age dating gives an approximate age of rocks, fossils, and events using uniformitarianism, original horizontality, superposition, cross-cutting relationships, and the principle of inclusion. Rock layers and the fossils that reside within them tell stories about their age and experiences. Part II Answer the Questions: Directions: Refer to the passage and pgs. 595-597 and pgs. 601-603 in your textbook to answer the questions below. 1. Compare and contrast absolute-age dating and relative-age dating below. 2. How does radiometric dating determine the exact age of rocks and fossils? ________________________________________________________ _________________________________________________________________________________________________________________________________________________ _________________________________________________________________________________________________________________________________________________ 3. Describe half-life. ________________________________________________________________________________________________________________________ _________________________________________________________________________________________________________________________________________________ 4. Define isotope. ____________________________________________________________________________________________________________________________ _________________________________________________________________________________________________________________________________________________ 5. Examine the rock layers in the picture on the left. List the rock layers in order by age. (oldest to youngest) __________________________________________________________________________________________________________________________ What could a scientist find in these layers to help determine their age? __________________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________________ Explain which relative-age dating technique scientist would use to determine the approximate age of these rock layers. __________________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________________ Part III Create a Scientific Model of Radioactive Decay Directions: In this activity you will make a scientific model of radioactive decay. Attempt to complete the activity BEFORE you ask for help. Parent Isotope = White Beans (use blue colored pencil) Daughter Element = Red-Brown Beans (use brown colored pencil) 1. Place 12 white beans in a bowl. This represents the parent isotope, which has just formed. Draw a picture of what the bowl looks like below. 2. Draw a circle graph showing how much of the material is made up of a parent isotope and daughter element. 3. Remove 6 of the white beans in the bowl and replace them with 6 red-brown beans. This represents what a material would be made up of after one half-life. Draw a picture of what the bowl looks like below. 4. Draw a circle graph showing how much of the material is made up of a parent isotope and daughter element. 5. Remove 3 of the white beans in the bowl and replace them with 3 red-brown beans. This represents what a material would be made up of after two half-lifes. Draw a picture of what the bowl looks like below. 6. Draw a circle graph showing how much of the material is made up of a parent isotope and daughter element. 7. After 3 half-lifes how many white and red-brown beans would be in the bowl? Draw a picture to show what you think the bowl would look like below. 8. Draw a circle graph showing how much of the material is made up of a parent isotope and daughter element after 3 halflifes.