Lesson 2 edTPA - WordPress.com
advertisement
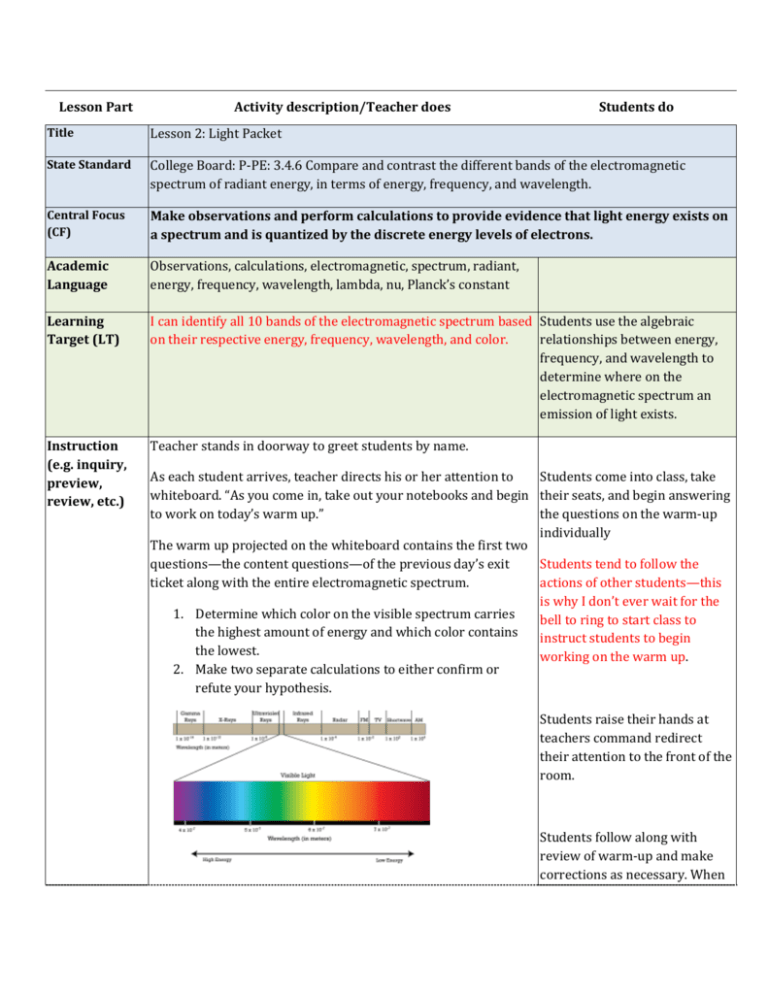
Lesson Part Activity description/Teacher does Students do Title Lesson 2: Light Packet State Standard College Board: P-PE: 3.4.6 Compare and contrast the different bands of the electromagnetic spectrum of radiant energy, in terms of energy, frequency, and wavelength. Central Focus (CF) Make observations and perform calculations to provide evidence that light energy exists on a spectrum and is quantized by the discrete energy levels of electrons. Academic Language Observations, calculations, electromagnetic, spectrum, radiant, energy, frequency, wavelength, lambda, nu, Planck’s constant Learning Target (LT) I can identify all 10 bands of the electromagnetic spectrum based Students use the algebraic on their respective energy, frequency, wavelength, and color. relationships between energy, frequency, and wavelength to determine where on the electromagnetic spectrum an emission of light exists. Instruction (e.g. inquiry, preview, review, etc.) Teacher stands in doorway to greet students by name. As each student arrives, teacher directs his or her attention to Students come into class, take whiteboard. “As you come in, take out your notebooks and begin their seats, and begin answering to work on today’s warm up.” the questions on the warm-up individually The warm up projected on the whiteboard contains the first two questions—the content questions—of the previous day’s exit Students tend to follow the ticket along with the entire electromagnetic spectrum. actions of other students—this is why I don’t ever wait for the 1. Determine which color on the visible spectrum carries bell to ring to start class to the highest amount of energy and which color contains instruct students to begin the lowest. working on the warm up. 2. Make two separate calculations to either confirm or refute your hypothesis. Students raise their hands at teachers command redirect their attention to the front of the room. Students follow along with review of warm-up and make corrections as necessary. When After seven minutes of class time has been given to work on the problem, teacher retrieves classroom attention by saying “Raise your hand if you can hear me.” Once all students are listening, teacher begins going over the warm-up. Teacher asks class, “Based on what we learned yesterday regarding the relationship between Energy, Frequency and wavelength, which color on the visible spectrum has the lowest energy?” prompted, students answer questions. Student who is called upon answers warm up question and explains how they reached their answer. If student provides an answer, teacher asks: “How did you come to that conclusion?” If student answers with the correct color and a rational explanation, teacher reaffirms student by saying they are correct, restates the student’s answer and explanation to the rest of the class and addresses a common misconception of the unit. “[Students name] is exactly right! As you can see on the visible spectrum, red is on the side of the visible spectrum with the longest wavelength—about 7x10-7m. And as should recall, energy is inversely proportional to wavelength. THIS IS THE BIGGEST MISCONCEPTION OF THIS ENTIRE UNIT! A lot of students get it worked in their heads that a long wave means more energy when in fact it’s the opposite. Think about the slinky demonstration from yesterday! The longer waves looked long and lazy in comparison to the short, fast waves. Remember: energy of a light wave depends on its frequency! Longer waves are simply less frequent. Make a note of that and put a huge box around it with some stars. Anything so it draws special attention Class makes corrections to their to itself. LONG WAVES = LOW ENERGY. Anyways, like [student warm up and makes a special name] said, if red is the lowest energy violet must be the highest. LONG WAVES=LOW ENERGY note in their notes. Teacher asks a different student at random what the relative length of a violet wave must be. “[student’s name], if violet has high energy, is its wave going to be longer or shorter than reds? Pause for answer… correct student if they are wrong and encourage them by saying that this really can be a confusing part of the light and energy sequence. Teacher finishes warm up coverage by conducting two calculations proving the work. Teacher explains that being able to relate wavelength to energy is an essential skill in answering this question. Further, that calculations can be completed to get an exact, quantitative amount of energy for each type of light by implementing the equation 𝐸 = ℎ𝑐 . 𝜆 “One way to check if you’re conceptualizing this right is to do the actual calculations—like #2 is asking you to do. Since we know the range of wavelengths for red and violet light, we can pick a band of each of them and make separate calculations. Combining E=hv and v=hc/𝜆 we can use the equation 𝐸 = ℎ𝑐 𝜆 to solve for the energy of a violet band as well as a red band.” Teacher conducts calculations and displays the answers: 4.97x10-19 J for violet 2.84x10-19 J for red This review should come from the fact that most students will have no idea how to do number two of the warm up. Based on information gathered from previous periods that day, there was a definite need for a math review. Students were having a very difficult time rearranging equations to find specific variables. That’s why instead of jumping straight into the warm up, I did a math review by relating 6=2x to 𝐸 = ℎ𝜈. Almost every student was comfortable with 6=2x, but when you turn numbers into variables students become uncomfortable. That’s why I walked them through that connection at the beginning of class. Teacher first asks if there are any questions about the warm up recap. Informal “Does anyone have any additional questions about what I just Assessment did?” Once questions have been asked and answered, teacher asks for a 0-5 to be held on their chests to assess how comfortable Students ask any questions that may have come up during the warm up recap. Students show the teacher 0-5 fingers by holding them on their chest for a private informal everyone in class was with the warm up. assessment. Teacher moves on if most students are a 4 or 5. If there is more than one student showing a 3 or lower, teacher verbally recounts everything he has written on the whiteboard through class so far, urging students to ask questions the moment they become confused. Practice Activity Support Teacher now divides students into new groups based on their exit tickets from the day before. Grouping will be based on: 1. Mastery of learning target from lesson 1—meaning students who circled 4’s or 5’s on meeting the learning target from the day before will be divided up evenly. As will students who circled 1’s and 2’s. 2. Classroom management. Teacher will assess which students work well together and which do not. Students begin working on the packet in newly assigned groups. Teacher explains that the rest of the period will be spent working on a group packet. Teacher explains every group member will receive the same score on the packet—encouraging collaboration. Teacher spends the majority of the remainder of class facilitating progress with the packet. Teacher believes in the power of the learning process so tries not to “helicopter” too closely above group work, but asks occasional prompting questions such as: Informal “How is everything going over here?” Assessment “Are things still running smoothly at this table?” “Are you guys having any trouble with any of the questions?” Otherwise, the teacher will stand back and wait to be summoned by a confused group or student. Students work together to complete the packet—using information given on the first page on the circulating teacher as resources. Practice Activity Support Through out the period, teacher periodically takes groups aside to complete #6 of the packet, which involves a hands on group activity where the class constructs the visible spectrum. Students pause their progress on packet and complete number 6 of the packet. 1. #6: In your groups, log onto ONE computer and go to this website: http://jersey.uoregon.edu/vlab/elements/Eleme nts.html. Once there, each member needs to click on their individually assigned element on the periodic table. Here you should see an absorption or emission spectrum. Click “emission” and select one band of light emitted by your element. On the piece of paper on the whiteboard in front of the room, draw this band in its correct location on the visible spectrum. Calculate the energy associated with its wavelength. On top of the band you draw, write the value of its wavelength. On the bottom of the band label its energy. Try and select your color based on what colors you don’t already see on the piece of paper and/or by having each group member choose a different color. Show the calculation of energy you made for the color you chose. This activity is what made this a two day packet. I couldn’t get all the groups to get through researching and drawing lines on the piece of paper. I need to find a different place to put this activity… and the earlier the better because it was actually really good having this thing hanging up in the classroom as a reference. Exit Ticket Closure One conceptual question and three student voice questions will Assessment conclude the day’s lesson. A completed (based on effort) exit ticket is the students’ ticket out the door. Students complete exit ticket and hand it to the teacher on their way out of the classroom.








