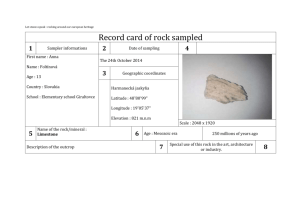
Manuscript_Van de Velde et al PRONEU-D-14-00098
advertisement

1 Towards axonal regeneration and neuroprotection in glaucoma: Rho kinase inhibitors as promising 2 therapeutics 3 Sarah Van de Velde1, Lies De Groef², Ingeborg Stalmans1, Lieve Moons2 and Inge Van Hove2 4 5 1 6 2 7 Section, Department of Biology, KU Leuven, Leuven, Belgium Laboratory of Ophthalmology, Department of Neurosciences, KU Leuven, Leuven, Belgium Neural Circuit Development and Regeneration Research Group, Animal Physiology and Neurobiology 8 9 Corresponding author: 10 Dr. Lieve Moons 11 Neural Circuit Development and Regeneration Research Group 12 Animal Physiology and Neurobiology Section 13 Department of Biology 14 KU Leuven 15 Naamsestraat 61, Box 2464 16 B-3000 Leuven, Belgium 17 Tel: +32-16-32.39.91 18 Fax: +32-16-32.42.62 19 Lieve.moons@bio.kuleuven.be 20 21 Running title: ROCK inhibition for retinal axonal regeneration and neuroprotection 22 Key words: ROCK inhibition, glaucoma, axonal regeneration, neuroprotection and ocular blood flow 23 1 24 Abstract 25 Due to a prolonged life expectancy worldwide, the incidence of age-related 26 neurodegenerative disorders such as glaucoma is increasing. Glaucoma is the second cause of 27 blindness, resulting from a slow and progressive loss of retinal ganglion cells (RGCs) and their axons. 28 Up to now, intraocular pressure (IOP) reduction is the only treatment modality by which 29 ophthalmologists attempt to control disease progression. However, not all patients benefit from this 30 therapy, and the pathophysiology of glaucoma is not always associated with an elevated IOP. These 31 limitations, together with the multifactorial etiology of glaucoma, urge the pressing medical need for 32 novel and alternative treatment strategies. Such new therapies should focus on preventing or 33 retarding RGC death, but also on repair of injured axons, to ultimately preserve or improve structural 34 and functional connectivity. In this respect, Rho-associated coiled-coil forming protein kinase (ROCK) 35 inhibitors hold a promising potential to become very prominent drugs for future glaucoma 36 treatment. Their field of action in the eye does not seem to be restricted to IOP reduction by 37 targeting the trabecular meshwork or improving filtration surgery outcome. Indeed, over the past 38 years, important progress has been made in elucidating their ability to improve ocular blood flow, to 39 prevent RGC death/increase RGC survival and to retard axonal degeneration or induce proper axonal 40 regeneration. Within this review, we aim to highlight the currently known capacity of ROCK inhibition 41 to promote neuroprotection and regeneration in several in vitro, ex vivo and in vivo experimental 42 glaucoma models. 43 44 Contents 45 I. Introduction .......................................................................................................................................... 3 46 II. Glaucoma pathophysiology ................................................................................................................. 4 47 III. Novel strategies for glaucoma therapy: neuroprotection and axonal regeneration ......................... 7 48 IV. The Rho-ROCK pathway ..................................................................................................................... 8 2 49 1. Downstream effectors of ROCK: well-known cytoskeletal regulators ............................................ 9 50 2. The ROCK signaling cascade and its involvement in neuronal cell death and axonal outgrowth . 10 51 V. ROCK inhibition as a multi-target approach for glaucoma treatment .............................................. 12 52 1. ROCK inhibitors as IOP lowering and anti-scarring agents in glaucoma ....................................... 12 53 2. ROCK inhibitors as novel neuroprotective and axon regenerative agents in glaucoma ............... 14 54 2.1. ROCK expression in the healthy and injured/diseased CNS ................................................... 15 55 2.2. ROCK inhibition as a promising tool to induce axon regeneration in the injured visual system 56 ....................................................................................................................................................... 16 57 2.3. ROCK inhibition as a promising tool to support neuronal survival in the injured visual system 58 ....................................................................................................................................................... 22 59 3. ROCK inhibition as promising regulators of ocular blood flow ..................................................... 26 60 V. Future perspectives: (pre-)clinical development of ROCK inhibitors for glaucoma treatment ........ 26 61 VI. Conclusion ........................................................................................................................................ 28 62 63 I. INTRODUCTION 64 The term glaucoma refers to a wide range of optic neuropathies associated with 65 degeneration of retinal ganglion cells (RGCs) and their respective axons, leading to slow progressive 66 visual field loss (Foster et al. 2002). If not adequately treated, most types of glaucoma progress 67 without obvious symptoms towards gradual loss of visual function or even blindness (Friedman et al. 68 2004). Nowadays, it is considered as the second leading cause of blindness throughout the world 69 (Resnikoff et al. 2008). This neurodegenerative disease mostly appears after the 4th decade of life, 70 and its frequency significantly increases with age. In 2010, at least 60.5 million people suffered from 71 glaucoma worldwide. This number is expected to increase up to 80 million by 2020 (Quigley and 3 72 Broman 2006). Risk factors for glaucoma not only include age, but also family history (Wolfs et al. 73 1998), gender (Rudnicka et al. 2006), ethnic background (Racette et al. 2003), severe myopia 74 (Mitchell et al. 1999), disturbed cerebrospinal fluid pressure (Fleischman and Allingham 2013), 75 vascular disorders (Bonomi et al. 2000) and importantly, an increased intraocular pressure (IOP) 76 (Boland and Quigley 2007). 77 With the currently available treatment modalities, glaucomatous visual field deterioration 78 cannot be prevented, yet lowering the IOP can slow down glaucoma disease progression 79 (VanVeldhuisen et al. 2000; Kass et al. 2002; Anastassiadis et al. 2011). Therefore glaucoma 80 treatment is mainly directed towards lowering IOP (VanVeldhuisen et al. 2000; Kass et al. 2002). A 81 sustained reduction of IOP can be achieved with topical therapy, laser therapy or surgical 82 interventions (Quigley 2011). Nevertheless, signs of progression can be seen in many patients despite 83 well-controlled IOP. Therefore, the development of neuroprotective strategies may be of high value 84 in the future treatment of this multifactorial neurodegenerative disorder. Until now, only two 85 potential neuroprotective agents have been investigated in clinical trials for glaucoma, i.e. 86 memantine and brimonidine. 87 Over the past few years, many studies have highlighted the important role of the Rho and 88 Rho-associated coiled-coil protein kinase (ROCK) pathway in the pathogenesis and treatment of 89 glaucoma. Modulation of this pathway seems to be involved in the regulation of IOP via the 90 trabecular meshwork (TM) and may also serve as a potent anti-scarring agent after glaucoma 91 surgery. More importantly, the amount of studies reporting ROCK inhibition as a very appealing 92 therapeutic approach that confers neuroprotection and axonal regeneration, substantially increased 93 over the last decade. Therefore, the purpose of this review is to summarize the current knowledge 94 on the upcoming neuroprotective and axon regenerative potential of ROCK inhibitors, which may be 95 of great importance in the development of a novel neuroprotective/regenerative strategy for 96 glaucoma therapy. 97 4 98 II. GLAUCOMA PATHOPHYSIOLOGY 99 The exact mechanisms of how glaucoma develops are still not well-known, although the 100 initiation and propagation of this disease is thought to be situated around the optic nerve head 101 (ONH). Yet, two principal hypotheses, the mechanical and ischemic/vascular theories have been 102 described (Fechtner and Weinreb 1994; Flammer et al. 2002b; Flammer and Mozaffarieh 2007) 103 (Figure 1). The classical mechanical theory suggests that the development of glaucoma is a direct 104 consequence of an increased IOP causing damage to the ONH (Yan et al. 1994). The IOP reflects the 105 balance between aqueous humor (AH) production and outflow through either the conventional 106 pathway via the TM, or the unconventional pathway via the uveoscleral route (Goel et al. 2010). IOP 107 elevation above 21 mmHg due to impaired TM function, is considered as the most important 108 measurable risk factor for the development of glaucoma (Weinreb and Khaw 2004) and believed to 109 induce damage to the RGC axons by tissue deformation at the level of the lamina cribrosa, thereby 110 leading to ONH cupping. When RGC axons exit the eye at the ONH, the lamina cribrosa provides the 111 only support and protection in an otherwise firm scleral shell, making it the most vulnerable site of 112 the retina/optic nerve to mechanical stress (Sigal and Ethier 2009). Cupping of the ONH in response 113 to elevated IOP causes compression of RGC axons, leading to a disruption of the axoplasmic transport 114 between the retina and the brain, which is essential for proper RGC survival (Flammer et al. 2002b). 115 Additionally, glial cells at the ONH shift their production of extracellular matrix (ECM) components 116 and increase their secretion of matrix metalloproteinases, thereby reducing mechanical support to 117 nerve fibers, making them even more vulnerable to elevated IOP (Hernandez 2000; Kirwan et al. 118 2004; Dahlmann-Noor et al. 2010; Quill et al. 2011; Akhter et al. 2013; De Groef et al. 2014). 119 Although generally accepted for a long time, the mechanical theory fails to explain several 120 features correlated with the appearance of glaucoma. Many patients (25-30%) suffer from 121 glaucomatous symptoms without the appearance of an increased IOP (normal tension glaucoma 122 (NTG)) and pressure reduction does not avoid glaucomatous damage in all patients with initial ocular 123 hypertension. On the other hand, some patients with ocular hypertension show no damage to the 5 124 optic nerve (Chauhan and Drance 1992; Martinez-Bello et al. 2000; Agarwal et al. 2009). These 125 observations indicate that multiple other factors, unrelated to IOP, play an important role in the 126 development of glaucoma. Increased incidence of glaucoma, especially NTG, is observed in patients 127 with systemic vascular disorders like hypotension (Hayreh et al. 1994; Hayreh 1999), cardiovascular 128 disease (Hayreh 1999), vasospastic disorders (Broadway and Drance 1998), migraine (Wang et al. 129 1997), diabetes (Mitchell et al. 1997) and cerebral ischemia (Stroman et al. 1995). Therefore, the 130 vascular theory considers a disturbed blood supply to the retina as the primary cause of optic 131 atrophy (Flammer et al. 2002b). Insufficient vascular autoregulation to adapt ocular perfusion may 132 result in a decreased retinal blood supply, causing ischemic damage (Morgan 2004). This then leads 133 to an impaired perfusion of the ONH, causing ischemic injury to the retina. Notably, the mechanical 134 and vascular theory are closely interlinked, because excavation of the ONH at the lamina cribrosa as 135 a direct consequence of elevated IOP, also leads to kinking of the retinal blood vessels (Flammer 136 1994; Flammer et al. 2002b; Flammer and Mozaffarieh 2007; Chang and Goldberg 2012b). 137 It is further hypothesized that both mechanical and/or ischemic insults to RGC axons 138 ultimately result in mitochondrial dysfunction leading to the induction of RGC apoptosis due to 139 oxidative damage (Figure 1). Mitochondrial dysfunction causes impaired energy supply, rendering 140 RGCs to exist in a lower energetic state, which results in a passive release of glutamate into the 141 extracellular space. In this state, cells are still able to transmit visual information, but are more 142 vulnerable to stress stimuli. Upon insult, also glial cells (microglia and astrocytes) will become 143 reactive, causing a gradual rise in cytokines and other factors, such as TNF-α, glutamate, 144 prostaglandins and nitric oxide. Initially, Müller glia will try to maintain a physiological balance, but 145 eventually, this process will become insufficient, resulting in an increased level of these molecules, 146 some of which, such as glutamate, are potentially toxic to neuronal cells (Toft-Kehler et al.2014). 147 Because RGCs are already in a decreased energetic state, they are more susceptible to be affected 148 and as a result, RGCs will undergo apoptosis at different rates, depending on their receptor profile 149 (Osborne et al. 2001; Osborne 2009; Chrysostomou et al. 2013). Notably, next to the mechanical and 6 150 vascular factors, inflammatory, autoimmune or even other (still unknown) factors may also 151 contribute to glaucoma pathophysiology. 152 III. NOVEL STRATEGIES FOR GLAUCOMA THERAPY: NEUROPROTECTION AND AXONAL REGENERATION 153 Neuroprotective strategies are extensively investigated in the context of several neurological 154 disorders, and form a therapeutic paradigm aiming at slowing down or preventing death of neurons. 155 In the case of glaucoma, where loss of RGCs progressively manifests over many years, a 156 neuroprotective therapy should enhance the survival of RGCs in the presence of chronic 157 stress/injury. Several approaches for neuroprotection in glaucoma are being investigated, targeting 158 neurotrophic withdrawal, excitotoxicity, oxidative stress, mitochondrial dysfunction, protein 159 misfolding, etc. (Baltmr et al. 2010). Given the importance of neurotrophins, such as e.g. brain 160 derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF) and nerve growth factor 161 (NGF), for the survival of RGCs (Weibel et al. 1995; Ji et al. 2004b), and the reduced axoplasmic 162 transport of these growth factors due to elevated IOP (Quigley et al. 2000), delivering neurotrophic 163 factors to the retina has been a key target in the development of neuroprotective therapies. Indeed, 164 multiple studies demonstrated the neuroprotective effect of BDNF, CNTF and NGF in animal models 165 of glaucoma (Johnson et al. 2011). These neurotrophic factor supplementation therapies are now 166 going into clinical trials for primary open angle glaucoma, e.g. trials with CNTF implants and NGF eye 167 drops (Chang and Goldberg 2012a), although the lack of methods for sustained, safe delivery of 168 biologically active neurotrophins hampers the broad clinical translation of this potential therapy. 169 Alternatively, neuroprotective agents preventing excitotoxicity, i.e. RGC apoptosis due to excessive 170 extracellular glutamate, have been investigated. This resulted in one of the only two clinical trials 171 evaluating neuroprotective agents for the treatment of glaucoma thus far. Indeed, memantine, a N- 172 methyl-D-aspartate (NMDA) receptor antagonist, prevents prolonged influx of Ca2+ ions through the 173 NMDA receptor channel and as such directly protects RGCs against excitotoxicity (Danesh-Meyer 174 2011). The other clinical trial, evaluating the use of the selective α2 receptor agonist brimonidine, 175 was based on the observation that this IOP lowering drug may protect RGCs from glaucomatous 7 176 neurodegeneration via a not yet known, IOP-independent mechanism (Lai et al. 2002; Dong et al. 177 2008). Nevertheless, despite good preclinical results, both clinical trials failed to deliver adequate 178 evidence for the applicability of memantine and brimonidine in the clinic, probably due to a too 179 narrow neuroprotective window of these agents (Weinreb 2007; Osborne 2009; Krupin et al. 2011). 180 Promoting survival of RGCs is a critical step in the development to a neuroprotective 181 strategy. However, in glaucoma also axons of RGCs are damaged, and as such, an ideal 182 neuroprotective glaucoma therapy should not only stimulate RGC survival but should also stimulate 183 axon regeneration (Chang and Goldberg 2012b). Clinical applications of the latter, however, seem to 184 be out of reach for now. For over three decades, the optic nerve crush (ONC) paradigm has been 185 used to study axonal regeneration of the optic nerve, first by stimulation of axonal outgrowth by 186 intraocular neurotrophic factor injection and peripheral nerve graft transplantation, and more 187 recently, by induction of controlled intraocular inflammation via experimental lens injury or 188 intravitreal zymosan injection. Moreover, manipulation of specific intracellular signaling pathways 189 has led to the identification of STAT3 (signal transducer and activator of transcription 3) and mTOR 190 (mammalian target of rapamycin) as central regulators in axonal outgrowth (Pernet and Schwab 191 2014). Despite these advances, it remains a challenge to induce sufficient axonal regeneration in 192 order to reinnervate retinal target areas in the brain, and restore visual function (de Lima et al. 2012; 193 Luo et al. 2013). 194 translatable to clinical practice. Moreover, the current experimental treatments in rodents are not readily 195 Of note, ‘vasoprotection’ could be added as an additional therapeutic approach to prevent 196 glaucomatous neurodegeneration (Wierzbowska et al. 2010), focusing on the prevention of ischemia 197 and reperfusion injury. Indeed, in a subset of patients, optic neuropathy is a consequence of vascular 198 dysregulation, due to e.g. systemic circulatory disturbances and perfusion deficits of cerebral, 199 retrobulbar and ocular vessels (Wierzbowska et al. 2010; Yanagi et al. 2011). Besides neuroprotective 200 drugs targeting excitotoxicity, these patients would greatly benefit from therapies restoring e.g. 8 201 vascular tone, endothelial function and neurovascular coupling (Flammer et al. 2002a; Yanagi et al. 202 2011). 203 IV. THE RHO-ROCK PATHWAY 204 Rho is a member of the cytoplasmic Rho family of small GTP-binding molecules, which 205 belongs to the Ras-superfamily of GTPases. The Rho subfamily contains 3 isoforms (RhoA, RhoB and 206 RhoC) that are ubiquitously expressed. To become activated, Rho must be targeted to the membrane 207 via posttranslational modification (Bishop and Hall 2000). The activity of the Rho pathway is 208 regulated by signaling input from several growth factors, cytokines, mechanical stretch and the ECM, 209 and as such mediated by different classes of cell surface receptors (Etienne-Manneville and Hall 210 2002). Rho GTPases function as molecular switches and cycle between an inactive GDP and an active 211 GTP-bound state. Activation is regulated by guanine nucleotide-exchange factors, which catalyze the 212 exchange of GDP to GTP. On the other hand, GTPase activating proteins inhibit downstream signaling 213 by stimulating the hydrolysis of GTP to GDP (Bos et al. 2007) (figure 2). The Rho-associated coiled- 214 coil-containing protein kinases (also known as ROCK) are the first identified and most extensively 215 studied downstream effectors of Rho. ROCKs are essentially distributed in the cytoplasm, but are 216 translocated to the plasma membrane after RhoA activation (Matsui et al. 1996). In mammals, ROCKs 217 exist as two isoforms: ROCKI and ROCKII. ROCKs contain three domains, the catalytic kinase domain, 218 which is located at the N-terminus, followed by a coiled-coil region containing the Rho-binding site 219 and a pleckstrin-homology domain with a cysteine-rich repeat domain at the carboxyl terminus 220 (Riento et al. 2003). The two ROCK isoforms share an overall homology in their sequences of 65%. 221 The highest similarities are found in their kinase domains, which are 92% identical. Both ROCK 222 isoforms are ubiquitously expressed in most tissues. However, there is a significant difference in 223 tissue distribution. Higher levels of ROCKII are found in brain and muscles, whereas ROCKI is 224 predominately expressed in non-neuronal tissues such as liver and lungs (Nakagawa et al. 1996). 9 225 1. Downstream effectors of ROCK: well-known cytoskeletal regulators 226 Once ROCK is activated by GTP-bound Rho, it is able to phosphorylate multiple substrates, 227 which almost all play a critical role in regulating cytoskeletal dynamics (Figure 2). The best- 228 characterized substrate of ROCK is the cytoskeletal regulator myosin light chain (MLC). 229 Phosphorylation of MLC results in stimulation of actin-myosin interactions, which play a role in 230 several cytoskeletal functions like cell morphology, adhesions, motility and smooth muscle cell 231 contraction. ROCK also regulates MLC activity via the inhibition of MLC phosphatase (Leung et al. 232 1996; Kaneko-Kawano et al. 2012). The actin-binding kinases, LIM kinase (LIMK) 1 and 2, are other 233 downstream targets of ROCK and are involved in the regulation of actin filament stabilization. Active 234 ROCK phosphorylates LIMK and thereby enhances its ability to phosphorylate cofilin, an actin-binding 235 and depolymerizing factor that regulates the turnover of actin filaments. Phosphorylation of cofilin 236 by LIMK inactivates its activity. Therefore, the phosphorylation of LIMK by ROCK inhibits cofilin- 237 mediated actin filament disassembly and leads to an increase in the number of actin filaments 238 (Ohashi et al. 2000; Sumi et al. 2001). ROCK also affects the ezrin/radixin/moesin (ERM) family, a 239 family of proteins that promote cross-linking of integral membrane proteins with actin filaments near 240 the cell surface. Phosphorylation and activation of the ERM proteins by ROCK regulates membrane- 241 actin interactions, permitting cytoskeletal reorganization and the establishment of focal adhesions 242 between the actin cytoskeletal and ECM (Tsukita and Yonemura 1997; Shaw et al. 1998). Another 243 ROCK effector is adducin, a filamentous-actin-binding protein which promotes actin network 244 assembly beneath the cell membrane and plays a role in cell motility (Matsuoka et al. 2000). 245 Intermediate filament proteins such as vimentin, glial fibrillary acidic protein (GFAP) and 246 neurofilaments, are cytoskeletal structures important for maintaining cell integrity and mechanical 247 strength, and their phosphorylation by ROCK causes intermediate filament disassembly and is 248 needed in specific events such as cytokinesis (Riento and Ridley 2003). ROCKs are also important 249 regulators of the process of inflammation, by the activation of NF-κβ, which subsequently controls 10 250 the transcription of proinflammatory factors such as interleukins and tumor necrosis factor-α, in 251 different inflammatory cells (Doe et al. 2007; He et al. 2008). 252 2. The ROCK signaling cascade and its involvement in neuronal cell death and axonal outgrowth 253 Several other downstream components of the ROCK pathway are highly expressed in the 254 CNS, and are regulators of several neural processes, ranging from neuronal survival to axonal 255 outgrowth and guidance (Schmandke and Strittmatter 2007) (Figure 2). 256 ROCK is a known negative regulator of neurite extension, resulting in axon retraction and 257 growth cone collapse during development and axon regeneration (Gallo 2006; Pasterkamp and 258 Verhaagen 2006). Indeed, ROCK is an important regulator of collapsin response mediator protein-2 259 (CRMP-2) that plays a role in semaphorin-3A-mediated repulsive axon guidance (Arimura et al. 2000). 260 Other downstream targets that play a major role in neurite extension are microtubule-associated 261 proteins (MAPs), such as Tau or MAP-2, which are important players in neuronal morphology by 262 altering microtubule dynamics (Amano et al. 2003). One of the most recently discovered and 263 interesting downstream targets of ROCK is the phosphatase and tensin homolog (PTEN). ROCK 264 phosphorylates and subsequently activates PTEN, thereby negatively regulating neuronal survival 265 and axonal regeneration via the PIP3/Akt/mTOR pathway (Li et al. 2005; Tonges et al. 2011). 266 Neurofilaments are intermediate proteins found in high concentrations along axons of vertebrate 267 neurons. Activation of these proteins by ROCK results in intermediate filament disassembly and as 268 such alters axon integrity and axonal growth dynamics (Hashimoto et al. 1998; Walker et al. 2001). 269 GFAP is another intermediate filament protein that is highly expressed in astrocytes and has been 270 shown to be important in repair after CNS injury and the formation of a glial scar (McKeon et al. 271 1991). Noteworthy, the Rho-ROCK pathway can be activated by several myelin-associated molecules 272 that inhibit axonal regeneration in the CNS, such as myelin-associated glycoprotein (MAG), neurite 273 outgrowth inhibitor (Nogo) and oligodendrocyte myelin glycoprotein (OMgp) (Hasegawa et al. 2004; 274 Mimura et al. 2006; Kubo et al. 2008). Indeed, the inability of mature CNS neurons to regenerate 275 injured axons has been attributed to a loss of an inherent growth potential of cells and to inhibitory 11 276 signals associated with myelin and the glial scar. These myelin-associated inhibitory molecules all 277 bind with high affinity the Nogo receptor (NgR) (McKerracher and Winton 2002). Interaction of this 278 receptor with the extracellular domain of p75NTR is known to activate Rho GTPase, and as such 279 activate ROCK, in turn resulting in neurite outgrowth inhibition and growth cone collapse (Kaplan and 280 Miller 2003; Yamashita and Tohyama 2003). Chondroitin sulfate proteoglycans (CSPGs), such as 281 brevican, aggrecan, versican, phosphocan and neuroglycan-2, are upregulated by reactive astrocytes 282 in the glial scar, formed around the lesion site. These CSPGs constitute an important family of 283 inhibitory ECM molecules (Silver and Miller 2004). It has been proposed that these CSPGs interact 284 with the ROCK pathway, resulting in growth inhibition (Gungabissoon and Bamburg 2003; Monnier et 285 al. 2003; Gopalakrishnan et al. 2008). 286 Thus, many of the known or suggested upstream and downstream signaling molecules of the 287 ROCK pathway in the CNS are related to axonal outgrowth after CNS damage, which subsequently 288 implicates the major contribution of the ROCK pathway to the restricted axonal regeneration in the 289 adult mammalian CNS. Blocking the Rho-ROCK signaling pathway is therefore yet another new 290 strategy to promote axonal regeneration and repair after injury in the CNS. 291 V. ROCK INHIBITION AS A MULTI-TARGET APPROACH FOR GLAUCOMA TREATMENT 292 Given the important role of Rho and ROCK in different cellular processes, targeting this 293 pathway may be of high value for various diseases. As such, inhibition of ROCK has already been 294 investigated as a potential strategy for cardiovascular diseases (Shi and Wei 2013), bronchial asthma 295 (Kume 2008) and cancer (Itoh et al. 1999). More recently, ROCK has also been highlighted as a 296 promising target for neurological disorders, as inhibition of ROCK seems to promote neuronal cell 297 survival and axonal regeneration (Mueller et al. 2005; Fujita and Yamashita 2014). 298 1. ROCK inhibitors as IOP lowering and anti-scarring agents in glaucoma 299 IOP lowering drugs currently in clinical use for glaucoma, suppress the production of AH or 300 enhance uveoscleral outflow. None of them directly targets and/or improves conventional outflow 12 301 via the TM. Therefore, agents that increase AH outflow by modifying the TM are promising targets 302 for the development of a new IOP lowering strategy to treat most forms of glaucoma (Llobet et al. 303 2003). The cells of the TM possess smooth muscle-like properties, as evidenced by the expression of 304 α-smooth muscle actin (α-SMA) (de Kater et al. 1990), and their contraction-relaxation status has 305 been reported to influence AH outflow facility (Wiederholt et al. 2000). Interestingly smooth muscle 306 cell contraction is regulated predominately by the phosphorylation status of MLC, a main 307 downstream target of ROCK. 308 In vitro and ex vivo studies have reported Rho and ROCK expression in healthy and 309 glaucomatous TM cells (Honjo et al. 2001a; Rao et al. 2001; Nakajima et al. 2005; Goldhagen et al. 310 2012). Cellular effects and IOP-lowering efficacy of several ROCK inhibitors, such as HA-1077, H- 311 1152P, K-115 Y27632, Y-39983 and AMA0076 have been previously reported (Honjo et al. 2001a; 312 Honjo et al. 2001b; Rao et al. 2001; Waki et al. 2001; Tokushige et al. 2007; Lu et al. 2008; Fukunaga 313 et al. 2009; Nishio et al. 2009; Isobe et al. 2014; Van de Velde et al. 2014a), and indeed unveiled that 314 ROCK regulates AH outflow by directly affecting contractility and cellular organization of the TM cells 315 (Honjo et al. 2001a; Rao et al. 2001). Overall, ROCK inhibition induces relaxation, actin cytoskeletal 316 reorganization and loss of cell adhesions of TM cells, leading to better drainage of AH trough the TM. 317 Not all patients respond adequately to anti-glaucoma medication making invasive therapies, 318 such as laser trabeculoplasty and/or filtration surgery, necessary for the treatment of glaucoma (Lee 319 and Higginbotham 2005). Unfortunately, filtration surgery frequently fails as the result of excessive 320 postoperative wound healing and subsequent scarring, leading to poor postoperative IOP control 321 and, as a consequence, progression of visual field loss (Addicks et al. 1983). During scar formation, 322 the cytokine TGF-β induces a contractile response in fibroblasts, which subsequently differentiate 323 into myofibroblasts. In vitro studies have shown that ROCK inhibitors might prevent this 324 transdifferentiation, probably via decreasing cell contraction, which implies that ROCK inhibitors may 325 reduce postoperative scarring after glaucoma filtration surgery (Meyer-ter-Vehn et al. 2006; Zhou et 326 al. 2013). Indeed, Honjo et al. showed that the ROCK inhibitor Y-27632 inhibited fibroproliferation 13 327 and collagen deposition in an animal model of glaucoma filtration surgery, leading to a prolonged 328 survival of the postoperative bleb (Honjo et al. 2007). 329 Thus, ROCK inhibition may possess therapeutic potential as a novel IOP lowering strategy and 330 be able to reduce postoperative scarring after filtration surgery, opening new perspectives for a 331 more efficient IOP control. 332 2. ROCK inhibitors as novel neuroprotective and axon regenerative agents in glaucoma 333 However, in NTG patients, glaucomatous damage occurs despite normal IOP levels and also 334 clinical evidence indicates that IOP lowering therapy does not prevent progression in all patients 335 (VanVeldhuisen et al. 2000). Since glaucomatous optic neuropathy eventually results in progressive 336 loss of RGCs, it is reasonable to expect that a neuroprotective therapy may be of high value as an 337 additional treatment modality for glaucoma. Such a therapy should not only directly promote RGC 338 survival, but should also encourage axonal regeneration, thereby recovering functional connections 339 (Chang and Goldberg 2012b). As we will discuss further in this review, there is increasing evidence 340 that ROCK is upregulated during glaucomatous damage and consequently, that ROCK inhibition 341 directly protects RGC survival and promotes axon regeneration. This emphasizes that ROCK inhibition 342 may be a versatile strategy for the treatment of glaucoma. 343 Much of the evidence concerning ROCK inhibition as a novel neuroprotective therapy has 344 been elucidated in glaucoma models (Kitaoka et al. 2004; Hirata et al. 2008; Lingor et al. 2008; Tura 345 et al. 2009; Alt et al. 2013). Furthermore, it has also been demonstrated that inhibition of ROCK limits 346 neurodegeneration in several in vitro, ex vivo and in vivo animal models for e.g. Amyotrophic Lateral 347 Sclerosis (ALS) (Tonges et al. 2014), Multiple Sclerosis (MS) (Zhang et al. ; Li et al. 2014a), 348 Huntington's (Li et al. 2009), Parkinson's (Tonges et al. 2012; Borrajo et al. 2014; Labandeira-Garcia et 349 al. 2014) and Alzheimer's disease (Mueller et al. 2005; Herskowitz et al. 2013; Song et al. 2013), 350 stroke/ischemia (Toshima et al. 2000; Satoh et al. 2001; Rikitake et al. 2005; Ding et al. 2009; 351 Koumura et al. 2011) and Spinal Muscular Atrophy (Bowerman et al. 2012; Froger et al. 2012). These 14 352 prevalent neurodegenerative disorders, which are characterized by a progressive loss of specific 353 neurons/axons, thereby leading to important dysfunctional consequences, still remain largely 354 incurable. Therefore, it is becoming increasingly clear that a therapeutic strategy with a 355 neuroprotective effect has an enormous future potential as effective treatment (Bull and Martin 356 2007; Cheung et al. 2008). 357 In the next paragraphs, this review will provide a detailed overview of the current knowledge 358 related to the potential of ROCK inhibition and the rationale why ROCK targeting seems a promising 359 tool to ensure neuronal protection and/or axonal regeneration. 360 2.1. ROCK expression in the healthy and injured/diseased CNS 361 The amount of studies describing ROCKI/II expression in the neuroretina is rather poor. In the 362 healthy rat retina, RhoA, ROCKI and ROCKII expression were reported to co-localize mainly in the 363 retinal microvessels (Arita et al. 2009). Another study could not demonstrate immunopositivity for 364 ROCKII in healthy rat retinas. However, axotomy of the rat optic nerve induced ROCKII expression, 365 but only in the RGC layer (RGCL), suggesting that ROCK might be detrimental to RGC survival and/or 366 contribute to a regeneration-inhibitory cascade (Lingor et al. 2008). Immunostainings for RhoA in rat 367 retinas also showed, after injection with the glutamate analog N-methyl-D-aspartate (NMDA), an 368 increased immunopositivity in the RGCL, the inner plexiform layer (IPL) and some somata in the inner 369 nuclear layer at 1 day post injection (1 dpi). Western blot data confirmed these findings and 370 demonstrated an upregulation of ROCKII in the NMDA-treated retina at 1 dpi, thereby suggesting a 371 commitment of Rho-ROCK signaling in NMDA/glutamate-induced retinal excitotoxicity (Kitaoka et al. 372 2004). Rho and ROCK expression in the ONH of glaucomatous patients is also significantly increased 373 as compared to healthy controls, indicating a possible involvement of the Rho-ROCK pathway in the 374 pathophysiology of optic nerve damage in glaucoma (Goldhagen et al. 2012). Clearly, additional 375 studies are necessary to investigate the cellular localization of ROCK, as well as its expression 376 patterns and activity levels in the healthy and glaucomatous retina, in order to understand the 15 377 inter/intracellular mechanisms contributing to RGC death and neurite outgrowth inhibition after 378 injury. 379 Studies in the healthy and diseased/injured brain and spinal cord reveal expression of ROCKII, 380 and to a much lower extent ROCKI, in neurons, endothelial cells and microglia (Nakagawa et al. 1996; 381 Deyts et al. 2009; Duffy et al. 2009). Moreover, treatment of PC12 cells with Nogo, a myelin- 382 associated neurite outgrowth inhibitor, did not only increase ROCKII activity, but also resulted in a 383 translocation of ROCKII towards the membrane (Alabed et al. 2006). This finding indicates that 384 ROCKII is activated by Nogo and strengthens the potential of ROCK inhibition in stimulating axonal 385 regeneration. 386 2.2. ROCK inhibition as a promising tool to induce axon regeneration in the injured visual system 387 An effective treatment to enable damaged axons to regrow beyond the lesion site and 388 reform functional connections should ideally require interventions targeting the cell-intrinsic growth 389 capacity, as well as the extrinsic inhibitory environment. In general, much progress concerning the 390 cellular and molecular mechanism underlying the failure of adult axons to regenerate, has been 391 made using studies in the rodent optic circuit. Functional recovery in the visual system implies the 392 proper regrowth of damaged axons to reinnervate the lateral geniculate nucleus and the superior 393 colliculus with sufficient numbers (Pernet et al. 2013; Murray 2014). Studies combining pten deletion 394 with either ‘suppressor of cytokine signaling 3’ (socs3) deletion, or zymosan and CPT-cAMP 395 injections, have demonstrated the potential of RGCs to reactivate their intrinsic axon growth 396 program and regenerate the full length of the optic nerve, across the optic chiasm (de Lima et al. 397 2012; Luo et al. 2013). Indeed, complete axonal regeneration to the lateral geniculate nucleus and 398 superior colliculus, along with partial recovery of visual functions, was observed upon pten deletion 399 plus CTP-cAMP and zymosan administration (de Lima et al. 2012). In an alternative approach, 400 combining pten and socs3 co-deletion with CNTF supplementation, short-distance axonal 401 regeneration to the suprachiasmatic nucleus was seen, yet no reinnervation of long-distance target 402 areas (Luo et al. 2013). Notably, adult RGCs were shown to be able to reactivate de novo axon growth 16 403 upon pten and socs3 co-deletion after injury, thereby making a future therapy to promote axon 404 repair more and more feasible. Yet, the poor number of RGC axons reaching their long-distance 405 target areas and the significant axon misguidance in the optic nerve and brain underscore the need 406 for further research efforts. 407 In vitro application of the ROCK inhibitors Y-27632, fasudil (HA-1077) and dimethylfasudil (H- 408 1152) on Ntera-2 neurons, PC12 cells and primary dorsal root ganglion cells, grown on a CSPG or 409 myelin substrate, was shown to overcome inhibition of neurite outgrowth (Fournier et al. 2003; 410 Zhang et al. 2006; Lingor et al. 2007; Cheng et al. 2008; Gopalakrishnan et al. 2008). It has also been 411 demonstrated in vivo that the amount, thickness and/or length of axons is increased after sciatic 412 nerve crush and spinal cord injury, and even locomotor activity, is improved after application of 413 ROCK inhibitors (Fournier et al. 2003; Sung et al. 2003; Hiraga et al. 2006; Cheng et al. 2008; Gunther 414 et al. 2014). Recently, inhibition of ROCK has proven to inhibit demyelination in an experimental 415 autoimmune encephalomyelitis animal model for MS (Gao et al. 2013). 416 As such, the potential of ROCK inhibitors to support axonal outgrowth and regeneration has 417 increased during the last years. Importantly, much evidence has been obtained from in vitro and ex 418 vivo studies on RGCs and retinal explants, and from experimental in vivo glaucoma models. 419 2.2.1. The neurite outgrowth-potential of ROCK inhibition in in vitro and ex vivo models for glaucoma 420 Addition of Y-27632, or the more potent Y-39983, to RGC-5 cells or postnatal rat primary 421 RGCs grown on a permissive substrate, demonstrated a clearly induced neurite outgrowth. Neurite 422 outgrowth was even more pronounced after combining Y-27632 with CNTF on these 2 cell cultures 423 (Lingor et al. 2008; Bermel et al. 2009; Tokushige et al. 2011) (Table 1). CNTF exerts its main 424 biological functions via activation of the Jak/STAT3, PI3K/Akt and MAPK/ERK signaling pathways (Park 425 et al. 2004), which are, interestingly, also activated upon ROCK inhibition (Lingor 2008, Pernet 2013, 426 Koch 2014). In general, STAT3 has been considered a central regulator of growth and regeneration of 427 adult RGCs and to promote their intrinsic growth capacity via transcription of growth-associated 428 genes and stabilization of the RGC cytoskeleton (Ng et al. 2006; Selvaraj et al. 2012; Pernet and 17 429 Schwab 2014). In contrast, STAT3 was once suggested to act as a negative regulator of neurite 430 outgrowth, proposing that Y-27632 may further increase neurite elongation in combination with 431 CNTF, by inhibiting STAT3 phosphorylation (Lingor et al. 2008). Furthermore, ROCK inhibition also 432 results in suppression of Lim kinase (LIMK) phosphorylation, which is induced upon binding of 433 myelin-derived inhibitory ligands (MAG, Nogo, OMgp) to their NgR receptor and leads to neurite 434 outgrowth inhibition and growth cone collapse (Sagawa et al. 2007). Similarly, ROCK inhibition can 435 relief the repulsive effects of glial-derived inhibitory ligands, such as CSPGs (Monnier et al. 2003). 436 Indeed, treatment of postnatal rat RGCs, grown on an inhibitory CSPG substrate, with Y-27632 437 significantly induced neurite length as compared to controls (Bermel et al. 2009). On the other hand, 438 treating adult rat retinal cells with a panel of inhibitory molecules, including Nogo-A, MAG, OMgp 439 and CSPG, prevented the stimulatory effect of Y-27632 on RGC neurite outgrowth (Ahmed et al. 440 2009) (Table 1). These, at first sight contradictory, findings might be explained by the age at which 441 the RGC cultures were obtained and might result from the suppressed 'growth activated state' of 442 adult CNS neurons, due to e.g. reduced cAMP levels, and as such, a limited regenerative capacity (Cai 443 et al. 2001). Remarkably, stimulation of adult retinal cells with Y-27632, in combination with CNTF, 444 significantly enhanced the number and length of RGC neurites, above the levels of CNTF treatment 445 alone. As such, Y-27632 does not seem to support neurite outgrowth of disinhibited adult RGCs, but 446 is able to enhance CNTF-induced neurite outgrowth (Ahmed et al. 2009). Similar findings were 447 observed when Y-27632-treated adult RGCs were supplemented with forskolin, which induces cAMP 448 levels. The combination of the three compounds enhanced neurite outgrowth of adult RGC neurites 449 to even greater levels than combined Y-27632 and forskolin treatment (Ahmed et al. 2009). 450 As the currently existing ROCK inhibitors do not distinguish between ROCKI and ROCKII, and 451 have been described to also block several other kinases and neuronal receptors (Mueller et al. 2005), 452 Koch et al. circumvented this issue by generating shRNA-expressing adeno-associated viral (AAV) 453 vectors to specifically downregulate ROCKII. Transduction of postnatal rat RGCs with AAV.ROCK2- 454 shRNA showed a trend towards longer neurites when grown on the permissive laminin substrate. 18 455 Moreover, knockdown of ROCKII enabled a full rescue of the growth-inhibiting effect of CSPG on 456 neurite outgrowth (Koch et al. 2014). 457 When embryonic rat retinal explants were applied in a stripe assay, consisting of alternate 458 laminin-coated and laminin/CSPG-coated stripes, or on a uniform laminin/CSPG substrate, axons 459 tended to avoid the CSPG substrate. Addition of Y-27632 clearly reduced this repulsive property and 460 significantly increased the amount and length of the outgrowing axons in comparison to non-treated 461 explants (Monnier et al. 2003) (Table 1). Administration of Y-27632 on adult rat retinal explants in 462 culture, dissected after optic nerve crush (ONC) (Herskowitz et al.), showed an increased number of 463 outgrowing neurites. When Y-27632 was applied together with Indolinone A, an inhibitor of cyclin 464 dependent kinase 5 (cdk5), which triggers a cascade of neurotoxic pathways in many 465 neurodegenerative disorders, an additional increase in outgrowth could be observed (Bermel et al. 466 2009). Supplementation of Y-27632 or Y-39983 also significantly induced neurite extension in adult 467 cat retinal explant studies (Sagawa et al. 2007; Ichikawa et al. 2008) (Table 1). Of note, the number of 468 extending neurites was higher when applying Y-39983, even at lower concentration than Y-27632, 469 thereby confirming the higher potency of Y-39983. Yet, no difference in neurite length could be 470 observed. Importantly, both Y-compounds also promoted the extension of glial processes in the cat 471 retinal explant assay (Sagawa et al. 2007). Notably, application of fasudil on adult cat retinal tissue 472 explants only induced glial fiber extension, while no neurite-outgrowth promoting effect was 473 observed (Ichikawa et al. 2008). 474 Using a retinal explant model, recently described by Buyens et al. (Buyens et al. 2014), our 475 group was able to demonstrate a clear neurite outgrowth-promoting effect of Y-27632 and Y-39883 476 in postnatal mouse retinal explants, respectively a 2 and 2.5 fold increase as compared to control 477 explants (Figure 3a) (Gaublomme et al. 2013). Also here, no significant neurite extension-promoting 478 effect was observed for any of the tested ROCK inhibitors (Figure 3b). Nevertheless, we also observed 479 a stimulating effect on glial process outgrowth (not shown). It is known that reactive glia, being 480 Müller glia and astrocytes, are present in the glaucomatous retina, although their exact role in the 19 481 disease pathogenesis seems to be ambiguous (Nickells 2007; Johnson and Morrison 2009). However, 482 their function as mediators of RGC survival and regeneration is being strengthened during the last 483 years and, amongst others, supported by their production of glial-derived growth factors (Muller et 484 al. 2007; Leibinger et al. 2009; Lorber et al. 2009). Next to soluble factors, also the expression of 485 membrane-bound glial derived growth factors, as well as axonal guidance molecules, contribute to 486 the, at least in part, RGC growth-promoting effect of activated retinal glia (Lorber et al. 2012). 487 These in vitro and ex vivo findings indicate that ROCK inhibitors have a strong capacity to 488 support axonal outgrowth, even in the presence of growth-inhibitory molecules, which mimics the in 489 vivo situation after axonal damage. 490 2.2.2. ROCK inhibitors promote in vivo axonal regeneration after optic nerve traumatic lesions 491 Intravitreal injection of Y-27632 or Y-39983 in the adult cat or rat, immediately after ONC, 492 promoted the number and length of regenerating axons passing the crush site, as compared to 493 crushed control eyes (Table 1). Also intravitreal injection of AAV.ROCK2-shRNA significantly increased 494 the number and length of growth associated protein 43 (GAP43)-positive neurites in the rat optic 495 nerve, after ONC, as compared to an AAV.EGFP-shRNA control vector (Koch et al. 2014). The 496 combined application of Y-27632 and the cdk5 inhibitor Indolinone A, resulted in a significantly 497 higher number, rather than an increased length, of regenerating axons beyond the ONC site, in 498 comparison to either compound alone (Bermel et al. 2009). On the other hand, administration of 499 different doses of fasudil or dimethylfasudil in a similar experimental set-up, revealed only minor 500 effects on axonal outgrowth (Lingor et al. 2007; Bermel et al. 2009) (Table 1). Repeated intravitreal 501 injections of Y-27632 in a rat sciatic nerve graft model, in which the sciatic nerve is transplanted onto 502 the optic nerve stump, did not result in additional axons in the nerve graft as compared to PBS- 503 treated eyes (Lingor et al. 2008) (Table 1). As peripheral nerves lack the major myelin-associated 504 inhibitory molecules, such as Nogo-A, this semi-permissive model allows to study the effect of 505 compounds on the intrinsic regenerative capacity of RGCs, as RGCs show a spontaneous regeneration 506 response into the graft (Thanos et al. 1997). The reported data would then suggest that inhibition of 20 507 ROCK rather promotes axon regeneration by modulating the extracellular environment at the injury 508 site than by altering the intrinsic growth capacity of RGCs. Anyway, co-application of Y-27632 and 509 CNTF enhanced the number of regenerating axons into the graft, to a greater extent than CNTF 510 alone, again indicating an advantageous interplay for these 2 molecules (Lingor et al. 2008). A 511 stronger regenerative response after combined administration of both compounds, as compared to 512 Y-27632 or CNTF treatment alone, was also observed in the (non-permissive) ONC model in the rat, 513 with a clearly more pronounced effect on the number, rather than the length, of regenerating axons 514 (Lingor et al. 2008). Importantly, also repeated injections of Y-27632 alone increased the number of 515 regenerating axons past the lesion site more than two-fold in comparison to control animals, thereby 516 reflecting the potential of ROCK inhibitors to overcome inhibitory myelin substrates. The adult rat 517 sciatic nerve graft model has also been applied to study the axon regenerative potential of Y-33983 518 (Tokushige et al. 2011) (Table 1). In comparison to the findings of Lingor et al. with Y-27632 (Lingor et 519 al. 2008), intravitreal injection and sponge application of solely Y-39983, dose-dependently increased 520 the number of RGCs with regenerating axons in peripheral nerve-transplanted retinas, as compared 521 to saline-administered eyes. Although Y-39983 is more potent than Y-27632 and might as such 522 induce axonal outgrowth to a higher extent (without CNTF), these two studies are difficult to 523 compare, as the amount and length of regenerated axons in the optic nerve was not determined in 524 the latter study (Tokushige et al. 2011). 525 Using a mouse ONC model and intravitreal injection of STAT3 cDNA-expressing AAV vectors, 526 Pernet et al. demonstrated that intracellular activation of STAT3 enhances axonal regeneration 527 (Pernet et al. 2013). However, STAT3-mediated axon elongation is characterized by massive 528 directionality and guidance problems, most likely due to the presence of growth-inhibitory molecules 529 in the optic nerve environment. Interestingly, additional intraocular delivery of Y-27632 did not only 530 result in an increased amount of outgrowing neurites (and surviving RGCs), but also counteracted the 531 Rho-LIMK-mediated misguidance effects and normalized the morphology and trajectories of the 532 regenerating axons. Intravitreal application of Y-27632 alone was not able to significantly induce 21 533 axonal regeneration. Importantly, combined delivery of Y-27632 and the Müller glia-selective AAV 534 vector ShH10.DH-CNTF, resulted in glial CNTF expression and activation of STAT3 in RGCs, eliciting a 535 more robust axonal outgrowth after ONC as compared to single ShH10.DH-CNTF or combined STAT3 536 and Y-27632 treatment (Pernet et al. 2013). These findings again support the notion that 537 combinatorial strategies will be needed. Indeed, co-injection of Y-27632 and AAV2.STAT3 raised 538 phosphorylated STAT3 (pSTAT3) levels and expression of STAT3 target genes, such as GAP43, after 539 injury (Pernet et al. 2013). 540 In summary, ROCK(II) inhibition is recently brought forward as a potential strategy to help to 541 overcome restriction of axonal regrowth in the injured CNS, such as the visual system. The amount of 542 studies on the RGC axon regenerative potential of ROCK inhibitors in animal models of glaucoma is 543 clearly increasing over the last years. As such, it seems that ROCK inhibitors might induce the number 544 of outgrowing axons, and help in a proper guidance of the axons towards their target areas, albeit in 545 combination with other neuroprotective and/or regenerative molecules. 546 2.3. ROCK inhibition as a promising tool to support neuronal survival in the injured visual system 547 Several studies highlighting the regenerative potential of ROCK inhibitors, also reported a 548 beneficial effect of ROCK inhibition on neuronal survival. However, the mechanisms responsible for 549 the neuroprotective effects of ROCK inhibitors in experimental models of neurodegenerative 550 disorders, are only gradually being elucidated over the past years. Modulation of the PTEN/Akt and 551 MAPK/ERK signaling pathways (Lingor et al. 2008; Tonges et al. 2012), a decrease in oxidative stress, 552 enhanced expression of neurotrophic factors (Li et al. 2014b) and attenuation of microglial activity 553 (Zhao et al. 2006; Suzuki et al. 2007; Borrajo et al. 2014) and CNS inflammatory responses (Ding et al. 554 2010; Villar-Cheda et al. 2012; Borrajo et al. 2014; Li et al. 2014a; Tonges et al. 2014), have all been 555 put forward as possible mechanisms underlying the observed increase in neuronal survival. 556 2.3.1. In vitro and ex vivo studies on the neuroprotective potential of ROCK inhibitors 557 Lingor et al. (2008) demonstrated that addition of Y-27632 significantly increased survival 558 and decreased apoptosis of serum-deprived RGC-5 cells and, although to a lesser extent, of 22 559 neurotrophin-deprived primary rat RGCs (Table 2). Combination of Y-27632 with CNTF in vitro, 560 resulted in a synergistic effect on RGC survival. CNTF has been repeatedly described to protect RGCs 561 in vitro and in vivo, in animal models of traumatic optic neuropathies and ocular hypertension 562 (Lehwalder et al. 1989; Mey and Thanos 1993; Weise et al. 2000; Ji et al. 2004a; Maier et al. 2004; 563 Park et al. 2004; Zhang et al. 2005). The neuroprotective effect of Y-27632 seems to be associated 564 with upregulated levels of phosphorylated MAPK, which becomes remarkably more phosphorylated 565 or activated after combined administration with CNTF, indicating that inhibition of ROCK activates 566 MAPK downstream signaling pathways that are also used by CNTF. pAkt levels were not altered after 567 Y-27632, but showed a clear trend towards upregulation after combination treatment. Addition of 568 CNTF onto RGC-5 cultures resulted in increased pSTAT3 levels. However, application of the ROCK 569 inhibitor alone, as well as the combination of both substances, did not alter or even decreased 570 pSTAT3 levels compared to CNTF treatment alone, indicating that ROCK weakens some CNTF 571 signaling pathways, while amplifying others such as MAPK and Akt pathways (Lingor et al. 2008). Also 572 Bermel et al. demonstrated increased survival of growth factor-deprived primary rat RGCs in culture 573 when supplemented with Y-27632 (Bermel et al. 2009) (Table 2). Combined application of Y-27632 574 and the cdk5 inhibitor Indolinone A promoted RGC survival to a higher extent than treatment with 575 either substance alone. Furthermore, and in accordance to the findings of Lingor et al. (Lingor et al. 576 2008), administration of Y-27632 to serum-deprived RGC-5 cells resulted in a minor increase in 577 pMAPK levels (Bermel et al. 2009). These in vitro observations indicate that, next to the MAPK 578 signaling pathway, additional survival promoting mechanisms mediate the neuroprotective effect of 579 ROCK inhibition. Importantly, the origin and nature of the RGC-5 cell line has been the subject of 580 controversy during recent years, and it is now recognized that it is in fact of mouse photoreceptor 581 origin (Krishnamoorthy et al. 2013; Sippl and Tamm 2014). Nevertheless, it is still widely used as a 582 tool to follow-up initial hypotheses that require a transformed retina cell line of neuronal origin 583 (Sippl and Tamm 2014). It is therefore imperative that findings are subsequently tested with 584 complementary, biologically relevant tools such as primary RGCs or ex vivo and in vivo models 23 585 (Krishnamoorthy et al. 2013; Sippl and Tamm 2014). Indeed, all RGC-5 data mentioned in this review 586 has been confirmed in more relevant biological backgrounds, as can readily be appreciated from 587 tables 1 and 2. 588 In addition to these cell culture studies, the effects of ROCK inhibition on RGC survival were 589 also investigated using different ex vivo models. Administration of dimethylfasudil (H-1152P) to 590 serum-deprived organotypic cultures of adult mouse retinas, significantly and dose-dependently 591 reduced cell death in the RGC layer (Tura et al. 2009) (Table 2). In addition, macro- and microglial 592 reactivity, which was dramatically elevated after serum deprivation, was clearly attenuated by 593 dimethylfasudil, and corresponded to the observed reduction in the cytokines levels produced by 594 these reactive glial cells. The neuroprotective potential of dimethylfasudil was confirmed in an ex 595 vivo model with perfused bovine retinas under hypoxia (Alt et al. 2013). Similarly, macro- and 596 microglial activities were suppressed after dimethylfasudil application, as demonstrated e.g. by the 597 preservation of quiescent, ramified microglia. These ex vivo observations suggest that inhibition of 598 ROCK is able to protect RGCs from cytotoxicity, an effect that might be mediated through 599 suppression of glial reactivity. 600 To conclude, several in vitro and ex vivo findings already point to the existence of ROCK 601 downstream signaling pathways affecting RGC survival. As ROCK inhibitors seem to possess the 602 ability to enhance neuroprotective capacities of other molecules, more in-depth insight into the 603 underlying working mechanisms and molecular players is needed. In addition, the effect of ROCK 604 inhibition on glial reactivity should be further considered to broaden our knowledge about the 605 complex actions of micro- and macroglia in neurodegeneration. 606 2.3.2. ROCK inhibitors as RGC survival-promoting molecules in animal models for glaucoma 607 Because of the involvement of RhoA in NMDA receptor signaling (Norenberg et al. 1999; 608 Nakazawa et al. 2003), a role of RhoA and ROCK in NMDA-induced retinal neurotoxicity was 609 presumed (Kitaoka et al. 2004). Co-administration of NMDA and fasudil diminished NMDA-induced 610 cell loss in the RGCL and prevented the reduction in IPL thickness, as compared to single NMDA 24 611 injected retinas, thereby proposing that inhibition of ROCK is neuroprotective against glutamate- 612 related excitotoxicity (Kitaoka et al. 2004)(Table 2). Also in a transient retinal ischemia model in rats, 613 cell loss in the RGCL and reduction of IPL thickness was diminished after intravitreal injection of Y- 614 27632 (Hirata et al. 2008). Moreover, disturbance of vascular endothelial cell alignment and 615 leukocyte infiltration was dramatically suppressed in the post-ischemic retina after Y-27632 616 administration, and might underlie the observed diminished neuronal cell death. Lingor et al. 617 demonstrated a neuroprotective role for Y-27632 after repeated intravitreal injections in the adult 618 rat eye after complete transection of the optic nerve (Lingor et al. 2008). The neuroprotective 619 capacities of dimethylfasudil were shown in an ONC model in the rat, where pretreatment with 620 dimethylfasudil reduced RGC apoptosis in a dose-dependent matter (Herskowitz et al.). Remarkably, 621 also here an additional attenuation of macro- and microglial reactivity was observed (Tura et al. 622 2009). An increased RGC survival was also observed in a mouse ONC model after daily oral treatment 623 with either fasudil or K-115, a novel ROCK inhibitor with high selectivity for ROCKII, as compared to 624 vehicle-treated mice (Yamamoto et al. 2014). Moreover, the mRNA levels of the RGC markers Thy1.2 625 and Brn3a were increased after K-115 or fasudil treatment. K-115 likely attenuates oxidative stress 626 and as such, retard RGC death and glaucoma progression, by reducing the levels of oxidized lipids, 627 reactive oxygen species and NAPDH oxidase 1, known to be involved in the ONC-induced ROS 628 production pathway (Yamamoto et al. 2014). Koch et al. (2014) showed significantly enhanced RGC 629 survival in an ONC model in rats after retinal transduction with AAV.ROCK2-shRNA. In addition, 630 axonal degeneration on the proximal side of the crush was clearly retarded after ROCKII inhibition. 631 Part of the specific ROCKII-inhibitory effects on RGC survival and axonal de- and regeneration, 632 observed after AAV.ROCK2-shRNA transduction, could probably be assigned to decreased 633 intraneuronal activity of calpain and caspase-3 and increased levels of pAkt and CRMP-2 and 634 autophagic flux (Koch et al. 2014). 635 The fact that ROCK(II) inhibition limits RGC apoptosis and supports RGC survival in various 636 animal models of glaucoma, points to its broad window of action, which might be beneficial and even 25 637 mandatory for the treatment of a complex multivariate disorder like glaucoma. Nevertheless, the 638 neuroprotective effect of ROCK inhibition is modest (~10% survival increase), and much lower in 639 comparison to e.g. CNTF of BDNF trophic support. 640 3. ROCK inhibition as promising regulators of ocular blood flow 641 Risk factors of vascular nature, leading to disturbed ocular perfusion, have been positively 642 correlated with the appearance of glaucoma. In some patients, mostly patients with NTG, vascular 643 disorders even play a predominate role. Therefore, drugs that improve ocular blood flow to the ONH 644 are promising as an additional treatment modality for glaucoma. 645 Although not studied in any detail yet, ROCK inhibitors were recently shown to affect ocular 646 blood flow. Tokushige et al. showed that topical administration of Y-39983 significantly increased 647 blood flow at the ONH in rabbit eyes (Tokushige et al. 2011). In another study, intravenous infusion 648 or topical treatment with fasudil suppressed the impairment and even improved ONH blood flow, 649 induced by a nitric oxide synthase inhibitor or endothelin-1 in rabbits (Sugiyama et al. 2011). These 650 blood flow-improving effects of ROCK inhibitors can be explained by the fact that the Rho-ROCK 651 pathway is involved in MLC regulated contraction of smooth muscle cells surrounding blood vessels 652 in the ONH. Indeed, activation of MLC is regulated by the counteracting enzymes MLC kinase and 653 MLC phosphatase and the activity of MLC phosphatase is inhibited by ROCK causing smooth muscle 654 cell contraction (Uehata et al. 1997). 655 Considering the role of vascular dysregulation and impaired ocular perfusion in the 656 pathogenesis of glaucoma, and especially in patients with NTG, these data indicate an additional 657 advantage of ROCK inhibitors as possible vasoprotective drugs for the treatment for glaucoma. 658 V. FUTURE PERSPECTIVES: (PRE-)CLINICAL DEVELOPMENT OF ROCK INHIBITORS FOR GLAUCOMA TREATMENT 659 Given the important role of ROCK signaling in numerous cellular processes, ROCK inhibitors 660 are of potential interest for the treatment of a variety of cardiovascular, inflammatory, autoimmune 661 and neurodegenerative diseases (Doe et al. 2007; Fernandes et al. 2007; Kast et al. 2007; Liao et al. 26 662 2007; LoGrasso and Feng 2009). Specifically for neurodegenerative diseases, spinal cord injury, optic 663 nerve injury, traumatic brain injury and stroke, Alzheimer’s, Parkinson’s and Huntington’s disease, 664 amyotrophic lateral sclerosis and multiple sclerosis have been named as potential indications. 665 Indeed, the improved neurological recovery after spinal cord lesion observed in a phase I/IIa clinical 666 trial with BA-210 (Cethrin, BioAxone) (Fehlings et al. 2011), illustrates that RhoA/ROCK inhibitors are 667 a rational therapeutic approach for the treatment of neurological disorders. Nevertheless, today only 668 one ROCK inhibitor, fasudil (Eril, Asahi Kasei Pharma), has been approved in Japan for the treatment 669 of cerebral vasospasm (Zhao et al. 2006; Suzuki et al. 2007). It is generally believed that this limited 670 success of clinical applications of ROCK inhibitors is due to their profound effect on blood pressure 671 (Kast et al. 2007). As a consequence, avoiding systemic exposure, recent development of ROCK 672 inhibitors has been largely restricted to topical applications, e.g. clinical trials with Y-39983 673 (Novartis), K-115 (Kowa Company), AR-12286, AR-13324 (Aerie Pharmaceuticals) and ATS907 674 (Altheos), to evaluate their IOP lowering effect. However, even when applied topically to the eye, 675 side effects such as conjunctival hyperemia are seen (Tokushige et al. 2007; Tanihara et al. 2008; 676 Mandell et al. 2011). Overall, most available ROCK inhibitors have a narrow therapeutic window, due 677 to adverse effects resulting from either their low specificity or their interference with off-target 678 ROCK-dependent cellular processes. While this limited specificity of current ROCK inhibitors, i.e. 679 inhibition of other protein kinases and lack of specificity for ROCK isoforms (Chen et al. 2011; Shi et 680 al. 2013), remains a concern, so-called ‘soft’ ROCK inhibitors (Amakem Therapeutics) have been 681 developed to overcome the second issue. These compounds, structurally related to Y-27632, are 682 locally acting drugs that are designed to be relatively stable at the site of action and undergo 683 metabolic inactivation by controlled conversion into a predictable, non-functional metabolite (Bodor 684 and Buchwald 2008). As a result, off-target activity is avoided and these compounds thus display a 685 unique pharmacokinetic profile and a superior safety profile (Boland et al. 2013). Indeed, topical 686 administration of AMA0076 potently lowered IOP in New Zealand white rabbits with minimal 687 hyperemia (Van de Velde et al. 2014b). Of note, as ROCKI and ROCKII have very distinct expression 27 688 profiles (Chen et al. 2011; Shi et al. 2013), the local mode of action of these soft ROCK inhibitors 689 implies that unwanted inhibition of the second ROCK isoform is dramatically reduced. In addition, 690 alternative pharmacological variants/classes are being generated and tested in various experimental 691 disease models, in order to improve kinase selectivity, cellular activity, microsomal stabilization and 692 pharmacokinetic properties of ROCK inhibitors (Kubo et al. 2008; Tonges et al. 2011). 693 VI. CONCLUSION 694 Since the adult mammalian CNS lacks the capacity to replace lost nerve cells or to regenerate 695 injured axons, neurodegenerative and neurotraumatic disorders seriously affect life quality and 696 human wellbeing. Also glaucoma, which is increasingly being recognized as a multifactorial disease, is 697 characterized by loss of functional RGCs, causing progressive visual field loss. IOP lowering therapies 698 still remain the mainstay of glaucoma therapy. However, considering IOP elevation as the only 699 treatable risk factor, is increasingly being challenged because some patients continue to have optic 700 nerve deterioration despite proper IOP control. This indicates that the need for complementary 701 approaches for the treatment of glaucoma is higher than ever. Considering glaucoma as a 702 neurodegenerative disease, the development of a neuroprotective strategy may provide improved 703 therapeutic options for all glaucoma patients. However, until now, and even despite good laboratory 704 evidence, no neuroprotective agent is yet available for glaucoma treatment. This could very well be 705 explained by the narrow window of their neuroprotective actions. This review summarizes 706 accumulating experimental evidence that ROCK inhibitors not only promote RGC survival, but also 707 RGC axon regeneration and ocular blood flow. Much of the progress that was recently made 708 concerning the potential of ROCK inhibition for neuroprotection and axonal regeneration has been 709 obtained from several (animal) models of glaucoma. These findings increased our knowledge on the 710 beneficial effects by which ROCK inhibitors support cell survival and axonal regrowth, and also 711 unveiled some contributing signaling pathways. In order to reach improved survival of injured 712 neurons and inhibition of repulsive environmental signaling in glaucoma, combinatorial, 713 complementary pharmacotherapies that target multiple pathogenic processes/signaling pathways 28 714 might have a higher potential than those targeting a single molecule. Therefore, it is important that 715 drug discovery work at the in vitro, ex vivo and in vivo level is being complemented by studies that 716 aim to elucidate the downstream signaling pathways by which ROCK inhibition establishes its effects. 717 Overall, to establish neuroprotection and axonal regeneration as effective treatment modalities in 718 order to improve long-term outcomes, potent, preferably locally acting, ROCK inhibitors may be a 719 novel pharmacological approach for glaucoma. 720 721 Conflict of interest 722 All authors report to have no conflict of interest. 723 724 Acknowledgments 725 The authors are financially supported by the Hercules Grant [AKUL/09/038] and national Grants from 726 the Research Council of KU Leuven [KU Leuven BOF-OT/10/033; GOA/12/008] and the Flemish 727 Institute for the promotion of scientific research (IWT and FWO). 728 29 729 Figures 730 731 Figure 1. Hypothetical cascade of events leading to RGC degeneration in glaucoma. 732 Both mechanical and/or ischemic insults may cause mitochondrial dysfunction, thereby resulting in 733 oxidative stress and subsequent RGC apoptosis due to oxidative damage. Mitochondrial dysfunction 734 compromises RGC energy supply rendering RGCs to exist in a lower energetic state. Upon insult, glial 735 cells will become reactivated causing a gradual release of growth factors and other molecules, such 736 as TNF-α, glutamate, prostaglandins (PGs) and nitric oxide (NO). Initially, Müller glia will try to 737 maintain a physiological balance, but eventually, this process will become insufficient. A gradual 738 increase in the level of these potential harmful molecules will exist and a toxic environment will be 739 established. Because RGCs are already in a decreased energetic state, they are even more susceptible 740 to further injury and as a result, also these RGCs will be triggered to death via apoptosis. 741 742 30 743 744 745 Figure 2. Downstream targets of ROCK. 746 Activation of ROCK by GTP-bound RhoA leads to phosphorylation of several substrates regulating a 747 diverse range of cellular responses. Most downstream targets of ROCKs are important cytoskeletal 748 regulators. Hence, the Rho-ROCK pathway has critical functions in cytoskeletal based activities such 749 as, cell adhesion, morphology, motility and contraction. Several other downstream effectors are 750 highly expressed in the central nervous system (CNS) (dotted lines), and are regulators of diverse 751 neural processes, ranging from neuronal survival to axon outgrowth and guidance (GDP: guanosine 752 diphosphate; GTP: guanosine triphosphate; GAP: GTP-ase activating proteins; GEF: guanine 753 nucleotide-exchange factors; ROCK: Rho-associated coiled-coil protein kinase; MLC: myosin light 754 chain; MLCP: MLC phosphatase; LIMK: LIM kinase; ERM: ezrin/radixin/moesin; MAP: microtubule- 755 associated protein; CRMP-2: collapsin response mediator protein-2; GFAP: glial fibrillary acidic 756 protein; NFs: neurofilament; PTEN: phosphatase and tensin homolog). 757 758 759 31 760 761 Figure 3. Effect of ROCK inhibitors on neurite outgrowth in mouse retinal explants. 762 (A) The neurite outgrowth-stimulating effect of Y-27632 (10µM) and Y-39983 (10µM) was 763 demonstrated in retinal explants, harvested from P3 mouse pups and immunostained for β-tubulin 764 after 3 days in culture, via automatic analysis of the immunostained neurite outgrowth area. 765 Outgrowth in control explants was set at 100%. A combinatorial treatment with the growth factors, 766 BDNF (50 ng/ml) and CNTF (10 ng/ml), was used as a positive control. Both ROCK inhibitors, but 767 mainly Y-39983, significantly promoted neurite outgrowth. (B) Neurite outgrowth extension was 768 investigated by categorizing neurite outgrowth into 4 segments, generated by successive 100 µm 769 increments in radius from the explant body edge and thus resulting in three ring segments, and an 770 outer segment covering the remaining immunolabeled neurites. BDNF/CNTF treatment clearly 771 stimulated axon extension, yet, no significant neurite-extension-promoting effect could be observed 772 for any of the ROCK inhibitors. Data are shown as mean ± SEM (n ≥ 34 for every condition, from 4 773 independent experiments; ** p < 0.01, *** p < 0.001, two-way ANOVA with Bonferroni post hoc 774 test). 32 Table 1. Overview of observed axon outgrowth/regenerative effects of ROCK inhibitors in experimental glaucoma models Drug Optimal Dosage form Model Animal Effects Reference reduced repulsive neurite outgrowth (Monnier et al. dose Y-27632 50µM E6 retinal explants chicken 2003) (+CSPG) Y-27632 10-100µM adult retinal explants cat Y-39983: more neurite outgrowth (Sagawa et al. Y-39983 3 & 10µM adult retinal explants cat similar effects on neurite length 2007) increased amount glial processes (Sagawa et al. 2007) Y-39983 10µM Gel foam + ONC cat increased amount and length of reg. axons (Sagawa et al. 2007) injection at crush + IV D0 (+7) Y-27632 1.5mM IV D0, 3, 6 ONC rat increased amount of reg. axons (Lingor et al. dimethylfasudil 4nM-40µM; IV D0, 3, 6 ONC rat no effect on axonal outgrowth 2007) (Lingor et al. 2007) 33 Y-27632 Y-27632 10µM RGC-5 cells 10µM P7-8 pRGCs enhanced neurite outgrowth (Lingor et al. rat enhanced neurite outgrowth 2008) 2.9mM IV D0, 3, 6 peri. nerve graft rat no effect on axon number 2.9mM IV D0, 3, 6 ONC rat increased amount reg. axons adult pRGCs rat no effect on neurite outgrowth in absence or (Ahmed et al. 10µM (+ myelin) Y-27632 30 & 100µM adult retinal explants presence of myelin cat increased amount of neurites & glia, no effect (Ichikawa et al. on process length fasudil 10 & 30µM adult retinal explants cat 2009) 2008) increased amount of glia, not neurites, no effect on length Y-27632 10 & 100µM IV D0, 7 + gel foam ONC cat increased amount and length of regenerating fasudil 10-100µM IV D0, 7 + gel foam ONC cat axons no regenerating axons (Ichikawa et al. 2008) (Ichikawa et al. 2008) (Ichikawa et al. 2008) 34 Y-27632 10µM RGC-5 cells 10µM P7-8 pRGCs (+ CSPG) 10µM adult retinal rat explants rat significantly increased neurite length (Bermel et al. enhanced neurite outgrowth 2009) increased amount of outgrowing axons after in vivo ONC 3.3µg Y-39983 Y-27632 IV D0, 3 10µM rat trend towards more reg. axons P7 pRGCs rat extended neurite outgrowth (Tokushige IV D0 + sponge peri. nerve graft rat increased amount regenerating axons al. 2011) 3mM IV D0, 7 ONC mouse no effect on axonal outgrowth (Pernet et al. P7 pRGCs rat enhanced neurite outgrowth 2013) ONC rat increased amount of reg. axons (Koch IV D-14 et 2014) Abbreviations: D: day of injection; pRGCs: primary retinal ganglion cells; ONC: optic nerve crush; RGCL: retinal ganglion cell layer, E: embryonic day; P: postnatal day; CSPG: chondroitin sulfate proteoglycan 35 et 10µM AAV.ROCK2shRNA ONC al. Table 2. Overview of observed neuroprotective effects of ROCK inhibitors in experimental glaucoma models Drug Optimal dose Y-27632 10µM Dosage form Model Animal Effects Reference P7-8 pRGCs (-NT) rat increased cell survival (Bermel et al. 2009) Y-27632 100nM IV, D0 transient retinal ischemia rat reduced cell loss in the RGCL (Hirata et increased IPL thickness al. 2008) diminished leukocyte infiltration & endothelial disarrangment Y-27632 (Lingor et rat increased RGC survival al. 2008) ONT rat increased RGC survival adult retinal explant cat no increased RGC survival RGC-5 (-serum) 10µM P7-8 pRGCs (-NTs) 2.9mM Y-39983 increased RGC survival 10µM IV, D0,3,6 10 & 30µM (Sagawa et al. 2007) Fasudil 10 & 100µM IV, D0 NMDA excitotoxicity rat reduced cell loss in the RGCL (Kitaoka et increased IPL thickness al. 2004) elevated Thy-1 mRNA levels 36 Fasudil K-115 1mg/kg 1mg/kg oral, daily oral, daily ONC mouse ONC mouse increased RGC survival increased Thy-1 mRNA levels (Yamamot increased RGC survival o reduced oxidative stress 2014) et al. (Yamamot o et al. 2014) dimethylfasudil 1µM adult retinal explant mouse (-serum) 1, 10 & 100µM IV D0 ONC rat reduced cell death (Tura et al. reduced macroglia reactivity 2009) reduced cell death in the RGCL reduced macro/microglia activity dimethylfasudil 1-5µM retinal explant (+ calf hypoxia) AAV.ROCK2shRNA IV D-14 rat ONC reduced cell death (Alt et al. reduced macro/microglia activity 2013) increased RGC survival (Koch et al. 2014) 37 Abbreviations: D: day of injection; pRGCs: primary retinal ganglion cells; NT: neurotrophins; P: postnatal day; ONT: optic nerve transection; ONC: optic nerve crush; RGCL: retinal ganglion cell layer; IPL: inner plexiform layer 38 References Addicks, E. M., Quigley, H. A., Green, W. R. and Robin, A. L. (1983). "Histologic characteristics of filtering blebs in glaucomatous eyes." Arch Ophthalmol 101(5): 795-798. Agarwal, R., Gupta, S. K., Agarwal, P., Saxena, R. and Agrawal, S. S. (2009). "Current concepts in the pathophysiology of glaucoma." Indian J Ophthalmol 57(4): 257-266. Ahmed, Z., Berry, M. and Logan, A. (2009). "ROCK inhibition promotes adult retinal ganglion cell neurite outgrowth only in the presence of growth promoting factors." Mol Cell Neurosci 42(2): 128-133. Akhter, N., Nix, M., Abdul, Y., Singh, S. and Husain, S. (2013). "Delta-opioid receptors attenuate TNFalpha-induced MMP-2 secretion from human ONH astrocytes." Invest Ophthalmol Vis Sci 54(10): 6605-6611. Alabed, Y. Z., Grados-Munro, E., Ferraro, G. B., Hsieh, S. H. and Fournier, A. E. (2006). "Neuronal responses to myelin are mediated by rho kinase." J Neurochem 96(6): 1616-1625. Alt, A., Hilgers, R. D., Tura, A., Nassar, K., Schneider, T., Hueber, A., Januschowski, K., Grisanti, S., Luke, J. and Luke, M. (2013). "The neuroprotective potential of Rho-kinase inhibition in promoting cell survival and reducing reactive gliosis in response to hypoxia in isolated bovine retina." Cell Physiol Biochem 32(1): 218-234. Amano, M., Kaneko, T., Maeda, A., Nakayama, M., Ito, M., Yamauchi, T., Goto, H., Fukata, Y., Oshiro, N., Shinohara, A., Iwamatsu, A. and Kaibuchi, K. (2003). "Identification of Tau and MAP2 as novel substrates of Rho-kinase and myosin phosphatase." J Neurochem 87(3): 780-790. Anastassiadis, T., Deacon, S. W., Devarajan, K., Ma, H. and Peterson, J. R. (2011). "Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity." Nat Biotechnol 29(11): 1039-1045. Arimura, N., Inagaki, N., Chihara, K., Menager, C., Nakamura, N., Amano, M., Iwamatsu, A., Goshima, Y. and Kaibuchi, K. (2000). "Phosphorylation of collapsin response mediator protein-2 by Rhokinase. Evidence for two separate signaling pathways for growth cone collapse." J Biol Chem 275(31): 23973-23980. Arita, R., Hata, Y., Nakao, S., Kita, T., Miura, M., Kawahara, S., Zandi, S., Almulki, L., Tayyari, F., Shimokawa, H., Hafezi-Moghadam, A. and Ishibashi, T. (2009). "Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage." Diabetes 58(1): 215-226. Baltmr, A., Duggan, J., Nizari, S., Salt, T. E. and Cordeiro, M. F. (2010). "Neuroprotection in glaucoma Is there a future role?" Exp Eye Res 91(5): 554-566. Bermel, C., Tonges, L., Planchamp, V., Gillardon, F., Weishaupt, J. H., Dietz, G. P., Bahr, M. and Lingor, P. (2009). "Combined inhibition of Cdk5 and ROCK additively increase cell survival, but not the regenerative response in regenerating retinal ganglion cells." Mol Cell Neurosci 42(4): 427-437. Bishop, A. L. and Hall, A. (2000). "Rho GTPases and their effector proteins." Biochem J 348 Pt 2: 241255. Bodor, N. and Buchwald, P. (2008). "Retrometabolic drug design: principles and recent developments." Pure Appl Chem 80(8): 1669-1682. Boland, M. V. and Quigley, H. A. (2007). "Risk factors and open-angle glaucoma: classification and application." J Glaucoma 16(4): 406-418. Boland, S., Defert, O., Alen, J., Bourin, A., Castermans, K., Kindt, N., Boumans, N., Panitti, L., Van de Velde, S., Stalmans, I. and Leysen, D. (2013). "3-[2-(Aminomethyl)-5-[(pyridin-4yl)carbamoyl]phenyl] benzoates as soft ROCK inhibitors." Bioorg Med Chem Lett. Bonomi, L., Marchini, G., Marraffa, M., Bernardi, P., Morbio, R. and Varotto, A. (2000). "Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study." Ophthalmology 107(7): 1287-1293. Borrajo, A., Rodriguez-Perez, A. I., Villar-Cheda, B., Guerra, M. J. and Labandeira-Garcia, J. L. (2014). "Inhibition of the microglial response is essential for the neuroprotective effects of Rhokinase inhibitors on MPTP-induced dopaminergic cell death." Neuropharmacology 85: 1-8. 39 Bos, J. L., Rehmann, H. and Wittinghofer, A. (2007). "GEFs and GAPs: critical elements in the control of small G proteins." Cell 129(5): 865-877. Bowerman, M., Murray, L. M., Boyer, J. G., Anderson, C. L. and Kothary, R. (2012). "Fasudil improves survival and promotes skeletal muscle development in a mouse model of spinal muscular atrophy." BMC medicine 10: 24. Broadway, D. C. and Drance, S. M. (1998). "Glaucoma and vasospasm." Br J Ophthalmol 82(8): 862870. Bull, N. D. and Martin, K. R. (2007). "Optic nerve restoration: new perspectives." J Glaucoma 16(5): 506-511. Buyens, T., Gaublomme, D., Van Hove, I., De Groef, L. and Moons, L. (2014). "Quantitative assessment of neurite outgrowth in mouse retinal explants." Methods Mol Biol 1162: 57-71. Cai, D., Qiu, J., Cao, Z., McAtee, M., Bregman, B. S. and Filbin, M. T. (2001). "Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate." J Neurosci 21(13): 47314739. Chang, E. E. and Goldberg, J. L. (2012b). "Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement." Ophthalmology 119(5): 979-986. Chauhan, B. C. and Drance, S. M. (1992). "The relationship between intraocular pressure and visual field progression in glaucoma." Graefes Arch Clin Exp Ophthalmol 230(6): 521-526. Chen, J., Runyan, S. A. and Robinson, M. R. (2011). "Novel ocular antihypertensive compounds in clinical trials." Clin Ophthalmol 5: 667-677. Cheng, C., Webber, C. A., Wang, J., Xu, Y., Martinez, J. A., Liu, W. Q., McDonald, D., Guo, G. F., Nguyen, M. D. and Zochodne, D. W. (2008). "Activated RHOA and peripheral axon regeneration." Exp Neurol 212(2): 358-369. Cheung, W., Guo, L. and Cordeiro, M. F. (2008). "Neuroprotection in glaucoma: drug-based approaches." Optom Vis Sci 85(6): 406-416. Chrysostomou, V., Rezania, F., Trounce, I. A. and Crowston, J. G. (2013). "Oxidative stress and mitochondrial dysfunction in glaucoma." Curr Opin Pharmacol 13(1): 12-15. Dahlmann-Noor, A. H., Vijay, S., Limb, G. A. and Khaw, P. T. (2010). "Strategies for optic nerve rescue and regeneration in glaucoma and other optic neuropathies." Drug Discov Today 15(7-8): 287-299. Danesh-Meyer, H. V. (2011). "Neuroprotection in glaucoma: recent and future directions." Curr Opin Ophthalmol 22(2): 78-86. De Groef, L., Van Hove, I., Dekeyster, E., Stalmans, I. and Moons, L. (2014). "MMPs in the neuroretina and optic nerve: modulators of glaucoma pathogenesis and repair?" Invest Ophthalmol Vis Sci 55(3): 1953-1964. de Kater, A. W., Spurr-Michaud, S. J. and Gipson, I. K. (1990). "Localization of smooth muscle myosincontaining cells in the aqueous outflow pathway." Invest Ophthalmol Vis Sci 31(2): 347-353. de Lima, S., Koriyama, Y., Kurimoto, T., Oliveira, J. T., Yin, Y., Li, Y., Gilbert, H. Y., Fagiolini, M., Martinez, A. M. and Benowitz, L. (2012). "Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors." Proc Natl Acad Sci U S A 109(23): 9149-9154. Deyts, C., Galan-Rodriguez, B., Martin, E., Bouveyron, N., Roze, E., Charvin, D., Caboche, J. and Betuing, S. (2009). "Dopamine D2 receptor stimulation potentiates PolyQ-Huntingtin-induced mouse striatal neuron dysfunctions via Rho/ROCK-II activation." PloS one 4(12): e8287. Ding, J., Li, Q. Y., Wang, X., Sun, C. H., Lu, C. Z. and Xiao, B. G. (2010). "Fasudil protects hippocampal neurons against hypoxia-reoxygenation injury by suppressing microglial inflammatory responses in mice." J Neurochem 114(6): 1619-1629. Ding, J., Yu, J. Z., Li, Q. Y., Wang, X., Lu, C. Z. and Xiao, B. G. (2009). "Rho kinase inhibitor Fasudil induces neuroprotection and neurogenesis partially through astrocyte-derived G-CSF." Brain Behav Immun 23(8): 1083-1088. Doe, C., Bentley, R., Behm, D. J., Lafferty, R., Stavenger, R., Jung, D., Bamford, M., Panchal, T., Grygielko, E., Wright, L. L., Smith, G. K., Chen, Z., Webb, C., Khandekar, S., Yi, T., Kirkpatrick, 40 R., Dul, E., Jolivette, L., Marino, J. P., Jr., Willette, R., Lee, D. and Hu, E. (2007). "Novel Rho kinase inhibitors with anti-inflammatory and vasodilatory activities." J Pharmacol Exp Ther 320(1): 89-98. Dong, C. J., Guo, Y., Agey, P., Wheeler, L. and Hare, W. A. (2008). "Alpha2 adrenergic modulation of NMDA receptor function as a major mechanism of RGC protection in experimental glaucoma and retinal excitotoxicity." Invest Ophthalmol Vis Sci 49(10): 4515-4522. Duffy, P., Schmandke, A., Sigworth, J., Narumiya, S., Cafferty, W. B. and Strittmatter, S. M. (2009). "Rho-associated kinase II (ROCKII) limits axonal growth after trauma within the adult mouse spinal cord." J Neurosci 29(48): 15266-15276. Etienne-Manneville, S. and Hall, A. (2002). "Rho GTPases in cell biology." Nature 420(6916): 629-635. Fechtner, R. D. and Weinreb, R. N. (1994). "Mechanisms of optic nerve damage in primary open angle glaucoma." Surv Ophthalmol 39(1): 23-42. Fehlings, M. G., Theodore, N., Harrop, J., Maurais, G., Kuntz, C., Shaffrey, C. I., Kwon, B. K., Chapman, J., Yee, A., Tighe, A. and McKerracher, L. (2011). "A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury." J Neurotrauma 28(5): 787-796. Fernandes, L. B., Henry, P. J. and Goldie, R. G. (2007). "Rho kinase as a therapeutic target in the treatment of asthma and chronic obstructive pulmonary disease." Ther Adv Respir Dis 1(1): 25-33. Flammer, J. (1994). "The vascular concept of glaucoma." Surv Ophthalmol 38 Suppl: S3-6. Flammer, J. and Mozaffarieh, M. (2007). "What is the present pathogenetic concept of glaucomatous optic neuropathy?" Surv Ophthalmol 52 Suppl 2: S162-173. Flammer, J., Orgül, S., Costa, V. P., Orzalesi, N., Krieglstein, G. K., Serra, L. M., Renard, J.-P. and Stefánsson, E. (2002a). "The impact of ocular blood flow in glaucoma." Prog Retin Eye Res 21(4): 359-393. Flammer, J., Orgul, S., Costa, V. P., Orzalesi, N., Krieglstein, G. K., Serra, L. M., Renard, J. P. and Stefansson, E. (2002b). "The impact of ocular blood flow in glaucoma." Prog Retin Eye Res 21(4): 359-393. Fleischman, D. and Allingham, R. R. (2013). "The role of cerebrospinal fluid pressure in glaucoma and other ophthalmic diseases: A review." Saudi J Ophthalmol 27(2): 97-106. Foster, P. J., Buhrmann, R., Quigley, H. A. and Johnson, G. J. (2002). "The definition and classification of glaucoma in prevalence surveys." Br J Ophthalmol 86(2): 238-242. Fournier, A. E., Takizawa, B. T. and Strittmatter, S. M. (2003). "Rho kinase inhibition enhances axonal regeneration in the injured CNS." J Neurosci 23(4): 1416-1423. Friedman, D. S., Wolfs, R. C., O'Colmain, B. J., Klein, B. E., Taylor, H. R., West, S., Leske, M. C., Mitchell, P., Congdon, N. and Kempen, J. (2004). "Prevalence of open-angle glaucoma among adults in the United States." Arch Ophthalmol 122(4): 532-538. Froger, N., Cadetti, L., Lorach, H., Martins, J., Bemelmans, A. P., Dubus, E., Degardin, J., Pain, D., Forster, V., Chicaud, L., Ivkovic, I., Simonutti, M., Fouquet, S., Jammoul, F., Leveillard, T., Benosman, R., Sahel, J. A. and Picaud, S. (2012). "Taurine provides neuroprotection against retinal ganglion cell degeneration." PLoS One 7(10): e42017. Fujita, Y. and Yamashita, T. (2014). "Axon growth inhibition by RhoA/ROCK in the central nervous system." Front Neurosci 8: 338. Fukunaga, T., Ikesugi, K., Nishio, M., Sugimoto, M., Sasoh, M., Hidaka, H. and Uji, Y. (2009). "The effect of the Rho-associated protein kinase inhibitor, HA-1077, in the rabbit ocular hypertension model induced by water loading." Curr Eye Res 34(1): 42-47. Gallo, G. (2006). "RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axon retraction." J Cell Sci 119(Pt 16): 3413-3423. Gao, C., Huang, L., Long, Y., Zheng, J., Yang, J., Pu, S. and Xie, L. (2013). "Y-39983, a selective Rhokinase inhibitor, attenuates experimental autoimmune encephalomyelitis via inhibition of demyelination." Neuroimmunomodulation 20(6): 334-340. Gaublomme, D., Buyens, T. and Moons, L. (2013). "Automated analysis of neurite outgrowth in mouse retinal explants." J Biomol Screen 18(5): 534-543. 41 Goel, M., Picciani, R. G., Lee, R. K. and Bhattacharya, S. K. (2010). "Aqueous humor dynamics: a review." Open Ophthalmol J 4: 52-59. Goldhagen, B., Proia, A. D., Epstein, D. L. and Rao, P. V. (2012). "Elevated levels of RhoA in the optic nerve head of human eyes with glaucoma." J Glaucoma 21(8): 530-538. Gopalakrishnan, S. M., Teusch, N., Imhof, C., Bakker, M. H., Schurdak, M., Burns, D. J. and Warrior, U. (2008). "Role of Rho kinase pathway in chondroitin sulfate proteoglycan-mediated inhibition of neurite outgrowth in PC12 cells." J Neurosci Res 86(10): 2214-2226. Gungabissoon, R. A. and Bamburg, J. R. (2003). "Regulation of growth cone actin dynamics by ADF/cofilin." J Histochem Cytochem 51(4): 411-420. Gunther, R., Saal, K. A., Suhr, M., Scheer, D., Koch, J. C., Bahr, M., Lingor, P. and Tonges, L. (2014). "The rho kinase inhibitor Y-27632 improves motor performance in male SOD1(G93A) mice." Front Neurosci 8: 304. Hasegawa, Y., Fujitani, M., Hata, K., Tohyama, M., Yamagishi, S. and Yamashita, T. (2004). "Promotion of axon regeneration by myelin-associated glycoprotein and Nogo through divergent signals downstream of Gi/G." J Neurosci 24(30): 6826-6832. Hashimoto, R., Nakamura, Y., Goto, H., Wada, Y., Sakoda, S., Kaibuchi, K., Inagaki, M. and Takeda, M. (1998). "Domain- and site-specific phosphorylation of bovine NF-L by Rho-associated kinase." Biochem Biophys Res Commun 245(2): 407-411. Hayreh, S. S. (1999). "The role of age and cardiovascular disease in glaucomatous optic neuropathy." Surv Ophthalmol 43 Suppl 1: S27-42. Hayreh, S. S., Zimmerman, M. B., Podhajsky, P. and Alward, W. L. (1994). "Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders." Am J Ophthalmol 117(5): 603-624. He, Y., Xu, H., Liang, L., Zhan, Z., Yang, X., Yu, X., Ye, Y. and Sun, L. (2008). "Antiinflammatory effect of Rho kinase blockade via inhibition of NF-kappaB activation in rheumatoid arthritis." Arthritis Rheum 58(11): 3366-3376. Hernandez, M. R. (2000). "The optic nerve head in glaucoma: role of astrocytes in tissue remodeling." Prog Retin Eye Res 19(3): 297-321. Herskowitz, J. H., Feng, Y., Mattheyses, A. L., Hales, C. M., Higginbotham, L. A., Duong, D. M., Montine, T. J., Troncoso, J. C., Thambisetty, M., Seyfried, N. T., Levey, A. I. and Lah, J. J. (2013). "Pharmacologic inhibition of ROCK2 suppresses amyloid-beta production in an Alzheimer's disease mouse model." J Neurosci 33(49): 19086-19098. Hiraga, A., Kuwabara, S., Doya, H., Kanai, K., Fujitani, M., Taniguchi, J., Arai, K., Mori, M., Hattori, T. and Yamashita, T. (2006). "Rho-kinase inhibition enhances axonal regeneration after peripheral nerve injury." J Peripher Nerv Syst 11(3): 217-224. Hirata, A., Inatani, M., Inomata, Y., Yonemura, N., Kawaji, T., Honjo, M. and Tanihara, H. (2008). "Y27632, a Rho-associated protein kinase inhibitor, attenuates neuronal cell death after transient retinal ischemia." Graefes Arch Clin Exp Ophthalmol 246(1): 51-59. Honjo, M., Inatani, M., Kido, N., Sawamura, T., Yue, B. Y., Honda, Y. and Tanihara, H. (2001a). "Effects of protein kinase inhibitor, HA1077, on intraocular pressure and outflow facility in rabbit eyes." Arch Ophthalmol 119(8): 1171-1178. Honjo, M., Tanihara, H., Inatani, M., Kido, N., Sawamura, T., Yue, B. Y., Narumiya, S. and Honda, Y. (2001b). "Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility." Invest Ophthalmol Vis Sci 42(1): 137-144. Honjo, M., Tanihara, H., Kameda, T., Kawaji, T., Yoshimura, N. and Araie, M. (2007). "Potential role of Rho-associated protein kinase inhibitor Y-27632 in glaucoma filtration surgery." Invest Ophthalmol Vis Sci 48(12): 5549-5557. Ichikawa, M., Yoshida, J., Saito, K., Sagawa, H., Tokita, Y. and Watanabe, M. (2008). "Differential effects of two ROCK inhibitors, Fasudil and Y-27632, on optic nerve regeneration in adult cats." Brain Res 1201: 23-33. Isobe, T., Mizuno, K., Kaneko, Y., Ohta, M., Koide, T. and Tanabe, S. (2014). "Effects of k-115, a rhokinase inhibitor, on aqueous humor dynamics in rabbits." Curr Eye Res 39(8): 813-822. 42 Itoh, K., Yoshioka, K., Akedo, H., Uehata, M., Ishizaki, T. and Narumiya, S. (1999). "An essential part for Rho-associated kinase in the transcellular invasion of tumor cells." Nat Med 5(2): 221225. Ji, J. Z., Elyaman, W., Yip, H. K., Lee, V. W., Yick, L. W., Hugon, J. and So, K. F. (2004a). "CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway." Eur J Neurosci 19(2): 265-272. Ji, J. Z., Elyaman, W., Yip, H. K., Lee, V. W., Yick, L. W., Hugon, J. and So, K. F. (2004b). "CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway." Eur J Neurosci 19(2): 265-272. Johnson, E. C. and Morrison, J. C. (2009). "Friend or foe? Resolving the impact of glial responses in glaucoma." J Glaucoma 18(5): 341-353. Johnson, T. V., Bull, N. D. and Martin, K. R. (2011). "Neurotrophic factor delivery as a protective treatment for glaucoma." Exp Eye Res 93(2): 196-203. Kaneko-Kawano, T., Takasu, F., Naoki, H., Sakumura, Y., Ishii, S., Ueba, T., Eiyama, A., Okada, A., Kawano, Y. and Suzuki, K. (2012). "Dynamic regulation of myosin light chain phosphorylation by Rho-kinase." PLoS One 7(6): e39269. Kaplan, D. R. and Miller, F. D. (2003). "Axon growth inhibition: signals from the p75 neurotrophin receptor." Nat Neurosci 6(5): 435-436. Kass, M. A., Heuer, D. K., Higginbotham, E. J., Johnson, C. A., Keltner, J. L., Miller, J. P., Parrish, R. K., 2nd, Wilson, M. R. and Gordon, M. O. (2002). "The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma." Arch Ophthalmol 120(6): 701-713; discussion 829-730. Kast, R., Schirok, H., Figueroa-Perez, S., Mittendorf, J., Gnoth, M. J., Apeler, H., Lenz, J., Franz, J. K., Knorr, A., Hutter, J., Lobell, M., Zimmermann, K., Munter, K., Augstein, K. H., Ehmke, H. and Stasch, J. P. (2007). "Cardiovascular effects of a novel potent and highly selective azaindolebased inhibitor of Rho-kinase." Br J Pharmacol 152(7): 1070-1080. Kirwan, R. P., Crean, J. K., Fenerty, C. H., Clark, A. F. and O'Brien, C. J. (2004). "Effect of cyclical mechanical stretch and exogenous transforming growth factor-beta1 on matrix metalloproteinase-2 activity in lamina cribrosa cells from the human optic nerve head." J Glaucoma 13(4): 327-334. Kitaoka, Y., Kumai, T., Lam, T. T., Kuribayashi, K., Isenoumi, K., Munemasa, Y., Motoki, M., Kobayashi, S. and Ueno, S. (2004). "Involvement of RhoA and possible neuroprotective effect of fasudil, a Rho kinase inhibitor, in NMDA-induced neurotoxicity in the rat retina." Brain Res 1018(1): 111-118. Koch, J. C., Tonges, L., Barski, E., Michel, U., Bahr, M. and Lingor, P. (2014). "ROCK2 is a major regulator of axonal degeneration, neuronal death and axonal regeneration in the CNS." Cell Death Dis 5: e1225. Koumura, A., Hamanaka, J., Kawasaki, K., Tsuruma, K., Shimazawa, M., Hozumi, I., Inuzuka, T. and Hara, H. (2011). "Fasudil and ozagrel in combination show neuroprotective effects on cerebral infarction after murine middle cerebral artery occlusion." J Pharmacol Exp Ther 338(1): 337-344. Krishnamoorthy, R. R., Clark, A. F., Daudt, D., Vishwanatha, J. K. and Yorio, T. (2013). "A forensic path to RGC-5 cell line identification: lessons learned." Invest Ophthalmol Vis Sci 54(8): 5712-5719. Krupin, T., Liebmann, J. M., Greenfield, D. S., Ritch, R. and Gardiner, S. (2011). "A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-Pressure Glaucoma Treatment Study." Am J Ophthalmol 151(4): 671-681. Kubo, T., Yamaguchi, A., Iwata, N. and Yamashita, T. (2008). "The therapeutic effects of Rho-ROCK inhibitors on CNS disorders." Ther Clin Risk Manag 4(3): 605-615. Kume, H. (2008). "RhoA/Rho-kinase as a therapeutic target in asthma." Curr Med Chem 15(27): 28762885. 43 Labandeira-Garcia, J. L., Rodriguez-Perez, A. I., Villar-Cheda, B., Borrajo, A., Dominguez-Meijide, A. and Guerra, M. J. (2014). "Rho Kinase and Dopaminergic Degeneration: A Promising Therapeutic Target for Parkinson's Disease." Neuroscientist Lai, R. K., Chun, T., Hasson, D., Lee, S., Mehrbod, F. and Wheeler, L. (2002). "Alpha-2 adrenoceptor agonist protects retinal function after acute retinal ischemic injury in the rat." Vis Neurosci 19(2): 175-185. Lee, D. A. and Higginbotham, E. J. (2005). "Glaucoma and its treatment: a review." Am J Health Syst Pharm 62(7): 691-699. Lehwalder, D., Jeffrey, P. L. and Unsicker, K. (1989). "Survival of purified embryonic chick retinal ganglion cells in the presence of neurotrophic factors." J Neurosci Res 24(2): 329-337. Leibinger, M., Muller, A., Andreadaki, A., Hauk, T. G., Kirsch, M. and Fischer, D. (2009). "Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor." J Neurosci 29(45): 14334-14341. Leung, T., Chen, X. Q., Manser, E. and Lim, L. (1996). "The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton." Mol Cell Biol 16(10): 5313-5327. Li, M., Huang, Y., Ma, A. A., Lin, E. and Diamond, M. I. (2009). "Y-27632 improves rotarod performance and reduces huntingtin levels in R6/2 mice." Neurobiol Dis 36(3): 413-420. Li, Y. H., Yu, J. Z., Liu, C. Y., Zhang, H., Zhang, H. F., Yang, W. F., Li, J. L., Feng, Q. J., Feng, L., Zhang, G. X., Xiao, B. G. and Ma, C. G. (2014a). "Intranasal delivery of FSD-C10, a novel Rho kinase inhibitor, exhibits therapeutic potential in experimental autoimmune encephalomyelitis." Immunology 143(2):219-229. Li, Z., Dong, X., Wang, Z., Liu, W., Deng, N., Ding, Y., Tang, L., Hla, T., Zeng, R., Li, L. and Wu, D. (2005). "Regulation of PTEN by Rho small GTPases." Nat Cell Biol 7(4): 399-404. Liao, J. K., Seto, M. and Noma, K. (2007). "Rho kinase (ROCK) inhibitors." J Cardiovasc Pharmacol 50(1): 17-24. Lingor, P., Teusch, N., Schwarz, K., Mueller, R., Mack, H., Bahr, M. and Mueller, B. K. (2007). "Inhibition of Rho kinase (ROCK) increases neurite outgrowth on chondroitin sulphate proteoglycan in vitro and axonal regeneration in the adult optic nerve in vivo." J Neurochem 103(1): 181-189. Lingor, P., Tonges, L., Pieper, N., Bermel, C., Barski, E., Planchamp, V. and Bahr, M. (2008). "ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells." Brain 131(Pt 1): 250-263. Llobet, A., Gasull, X. and Gual, A. (2003). "Understanding trabecular meshwork physiology: a key to the control of intraocular pressure?" News Physiol Sci 18: 205-209. LoGrasso, P. V. and Feng, Y. (2009). "Rho kinase (ROCK) inhibitors and their application to inflammatory disorders." Curr Top Med Chem 9(8): 704-723. Lorber, B., Berry, M., Douglas, M. R., Nakazawa, T. and Logan, A. (2009). "Activated retinal glia promote neurite outgrowth of retinal ganglion cells via apolipoprotein E." J Neurosci Res 87(12): 2645-2652. Lorber, B., Guidi, A., Fawcett, J. W. and Martin, K. R. (2012). "Activated retinal glia mediated axon regeneration in experimental glaucoma." Neurobiol Dis 45(1): 243-252. Lu, Z., Overby, D. R., Scott, P. A., Freddo, T. F. and Gong, H. (2008). "The mechanism of increasing outflow facility by rho-kinase inhibition with Y-27632 in bovine eyes." Exp Eye Res 86(2): 271281. Luo, X., Salgueiro, Y., Beckerman, S. R., Lemmon, V. P., Tsoulfas, P. and Park, K. K. (2013). "Threedimensional evaluation of retinal ganglion cell axon regeneration and pathfinding in whole mouse tissue after injury." Exp Neurol 247: 653-662. Maier, K., Rau, C. R., Storch, M. K., Sattler, M. B., Demmer, I., Weissert, R., Taheri, N., Kuhnert, A. V., Bahr, M. and Diem, R. (2004). "Ciliary neurotrophic factor protects retinal ganglion cells from 44 secondary cell death during acute autoimmune optic neuritis in rats." Brain Pathol 14(4): 378-387. Mandell, K. J., Kudelka, R. M. and Wirostko, B. (2011). "Rho kinase inhibitors for treatment of glaucoma." Expert Rev Ophthalmol 6(6): 611-622. Martinez-Bello, C., Chauhan, B. C., Nicolela, M. T., McCormick, T. A. and LeBlanc, R. P. (2000). "Intraocular pressure and progression of glaucomatous visual field loss." Am J Ophthalmol 129(3): 302-308. Matsui, T., Amano, M., Yamamoto, T., Chihara, K., Nakafuku, M., Ito, M., Nakano, T., Okawa, K., Iwamatsu, A. and Kaibuchi, K. (1996). "Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho." EMBO J 15(9): 2208-2216. Matsuoka, Y., Li, X. and Bennett, V. (2000). "Adducin: structure, function and regulation." Cell Mol Life Sci 57(6): 884-895. McKeon, R. J., Schreiber, R. C., Rudge, J. S. and Silver, J. (1991). "Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes." J Neurosci 11(11): 3398-3411. McKerracher, L. and Winton, M. J. (2002). "Nogo on the go." Neuron 36(3): 345-348. Mey, J. and Thanos, S. (1993). "Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo." Brain Res 602(2): 304-317. Meyer-ter-Vehn, T., Sieprath, S., Katzenberger, B., Gebhardt, S., Grehn, F. and Schlunck, G. (2006). "Contractility as a prerequisite for TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts." Invest Ophthalmol Vis Sci 47(11): 4895-4904. Mimura, F., Yamagishi, S., Arimura, N., Fujitani, M., Kubo, T., Kaibuchi, K. and Yamashita, T. (2006). "Myelin-associated glycoprotein inhibits microtubule assembly by a Rho-kinase-dependent mechanism." J Biol Chem 281(23): 15970-15979. Mitchell, P., Hourihan, F., Sandbach, J. and Wang, J. J. (1999). "The relationship between glaucoma and myopia: the Blue Mountains Eye Study." Ophthalmology 106(10): 2010-2015. Mitchell, P., Smith, W., Chey, T. and Healey, P. R. (1997). "Open-angle glaucoma and diabetes: the Blue Mountains eye study, Australia." Ophthalmology 104(4): 712-718. Monnier, P. P., Sierra, A., Schwab, J. M., Henke-Fahle, S. and Mueller, B. K. (2003). "The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar." Mol Cell Neurosci 22(3): 319-330. Morgan, J. E. (2004). "Circulation and axonal transport in the optic nerve." Eye (Lond) 18(11): 10891095. Mueller, B. K., Mack, H. and Teusch, N. (2005). "Rho kinase, a promising drug target for neurological disorders." Nat Rev Drug Discov 4(5): 387-398. Muller, A., Hauk, T. G. and Fischer, D. (2007). "Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation." Brain 130(Pt 12): 3308-3320. Murray, A. J. (2014). "Axon regeneration: what needs to be overcome?" Methods Mol Biol 1162: 314. Nakagawa, O., Fujisawa, K., Ishizaki, T., Saito, Y., Nakao, K. and Narumiya, S. (1996). "ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice." FEBS Lett 392(2): 189-193. Nakajima, E., Nakajima, T., Minagawa, Y., Shearer, T. R. and Azuma, M. (2005). "Contribution of ROCK in contraction of trabecular meshwork: proposed mechanism for regulating aqueous outflow in monkey and human eyes." J Pharm Sci 94(4): 701-708. Nakazawa, T., Watabe, A. M., Tezuka, T., Yoshida, Y., Yokoyama, K., Umemori, H., Inoue, A., Okabe, S., Manabe, T. and Yamamoto, T. (2003). "p250GAP, a novel brain-enriched GTPaseactivating protein for Rho family GTPases, is involved in the N-methyl-d-aspartate receptor signaling." Mol Biol Cell 14(7): 2921-2934. Ng, D. C., Lin, B. H., Lim, C. P., Huang, G., Zhang, T., Poli, V. and Cao, X. (2006). "Stat3 regulates microtubules by antagonizing the depolymerization activity of stathmin." J Cell Biol 172(2): 245-257. 45 Nickells, R. W. (2007). "From ocular hypertension to ganglion cell death: a theoretical sequence of events leading to glaucoma." Can J Ophthalmol 42(2): 278-287. Nishio, M., Fukunaga, T., Sugimoto, M., Ikesugi, K., Sumi, K., Hidaka, H. and Uji, Y. (2009). "The effect of the H-1152P, a potent Rho-associated coiled coil-formed protein kinase inhibitor, in rabbit normal and ocular hypertensive eyes." Curr Eye Res 34(4): 282-286. Norenberg, W., Hofmann, F., Illes, P., Aktories, K. and Meyer, D. K. (1999). "Rundown of somatodendritic N-methyl-D-aspartate (NMDA) receptor channels in rat hippocampal neurones: evidence for a role of the small GTPase RhoA." Br J Pharmacol 127(5): 1060-1063. Ohashi, K., Nagata, K., Maekawa, M., Ishizaki, T., Narumiya, S. and Mizuno, K. (2000). "Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop." J Biol Chem 275(5): 3577-3582. Osborne, N. N. (2009). "Recent clinical findings with memantine should not mean that the idea of neuroprotection in glaucoma is abandoned." Acta Ophthalmol 87(4): 450-454. Osborne, N. N., Melena, J., Chidlow, G. and Wood, J. P. (2001). "A hypothesis to explain ganglion cell death caused by vascular insults at the optic nerve head: possible implication for the treatment of glaucoma." Br J Ophthalmol 85(10): 1252-1259. Park, K., Luo, J. M., Hisheh, S., Harvey, A. R. and Cui, Q. (2004). "Cellular mechanisms associated with spontaneous and ciliary neurotrophic factor-cAMP-induced survival and axonal regeneration of adult retinal ganglion cells." J Neurosci 24(48): 10806-10815. Pasterkamp, R. J. and Verhaagen, J. (2006). "Semaphorins in axon regeneration: developmental guidance molecules gone wrong?" Philos Trans R Soc Lond B Biol Sci 361(1473): 1499-1511. Pernet, V., Joly, S., Jordi, N., Dalkara, D., Guzik-Kornacka, A., Flannery, J. G. and Schwab, M. E. (2013). "Misguidance and modulation of axonal regeneration by Stat3 and Rho/ROCK signaling in the transparent optic nerve." Cell Death Dis 4: e734. Pernet, V. and Schwab, M. E. (2014). "Lost in the jungle: new hurdles for optic nerve axon regeneration." Trends Neurosci 37(7): 381-387. Quigley, H. A. (2011). "Glaucoma." Lancet 377(9774): 1367-1377. Quigley, H. A. and Broman, A. T. (2006). "The number of people with glaucoma worldwide in 2010 and 2020." Br J Ophthalmol 90(3): 262-267. Quigley, H. A., McKinnon, S. J., Zack, D. J., Pease, M. E., Kerrigan-Baumrind, L. A., Kerrigan, D. F. and Mitchell, R. S. (2000). "Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats." Invest Ophthalmol Vis Sci 41(11): 3460-3466. Quill, B., Docherty, N. G., Clark, A. F. and O'Brien, C. J. (2011). "The effect of graded cyclic stretching on extracellular matrix-related gene expression profiles in cultured primary human lamina cribrosa cells." Invest Ophthalmol Vis Sci 52(3): 1908-1915. Racette, L., Wilson, M. R., Zangwill, L. M., Weinreb, R. N. and Sample, P. A. (2003). "Primary openangle glaucoma in blacks: a review." Surv Ophthalmol 48(3): 295-313. Rao, P. V., Deng, P. F., Kumar, J. and Epstein, D. L. (2001). "Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632." Invest Ophthalmol Vis Sci 42(5): 10291037. Resnikoff, S., Pascolini, D., Mariotti, S. P. and Pokharel, G. P. (2008). "Global magnitude of visual impairment caused by uncorrected refractive errors in 2004." Bull World Health Organ 86(1): 63-70. Riento, K., Guasch, R. M., Garg, R., Jin, B. and Ridley, A. J. (2003). "RhoE binds to ROCK I and inhibits downstream signaling." Mol Cell Biol 23(12): 4219-4229. Riento, K. and Ridley, A. J. (2003). "Rocks: multifunctional kinases in cell behaviour." Nat Rev Mol Cell Biol 4(6): 446-456. Rikitake, Y., Kim, H. H., Huang, Z., Seto, M., Yano, K., Asano, T., Moskowitz, M. A. and Liao, J. K. (2005). "Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection." Stroke 36(10): 2251-2257. 46 Rudnicka, A. R., Mt-Isa, S., Owen, C. G., Cook, D. G. and Ashby, D. (2006). "Variations in primary openangle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis." Invest Ophthalmol Vis Sci 47(10): 4254-4261. Sagawa, H., Terasaki, H., Nakamura, M., Ichikawa, M., Yata, T., Tokita, Y. and Watanabe, M. (2007). "A novel ROCK inhibitor, Y-39983, promotes regeneration of crushed axons of retinal ganglion cells into the optic nerve of adult cats." Exp Neurol 205(1): 230-240. Satoh, S., Utsunomiya, T., Tsurui, K., Kobayashi, T., Ikegaki, I., Sasaki, Y. and Asano, T. (2001). "Pharmacological profile of hydroxy fasudil as a selective rho kinase inhibitor on ischemic brain damage." Life Sci 69(12): 1441-1453. Schmandke, A. and Strittmatter, S. M. (2007). "ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases." Neuroscientist 13(5): 454-469. Selvaraj, B. T., Frank, N., Bender, F. L., Asan, E. and Sendtner, M. (2012). "Local axonal function of STAT3 rescues axon degeneration in the pmn model of motoneuron disease." J Cell Biol 199(3): 437-451. Shaw, R. J., Henry, M., Solomon, F. and Jacks, T. (1998). "RhoA-dependent phosphorylation and relocalization of ERM proteins into apical membrane/actin protrusions in fibroblasts." Mol Biol Cell 9(2): 403-419. Shi, J. and Wei, L. (2013). "Rho kinases in cardiovascular physiology and pathophysiology: the effect of fasudil." J Cardiovasc Pharmacol 62(4): 341-354. Shi, J., Wu, X., Surma, M., Vemula, S., Zhang, L., Yang, Y., Kapur, R. and Wei, L. (2013). "Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment." Cell Death Dis 4: e483. Sigal, I. A. and Ethier, C. R. (2009). "Biomechanics of the optic nerve head." Exp Eye Res 88(4): 799807. Silver, J. and Miller, J. H. (2004). "Regeneration beyond the glial scar." Nat Rev Neurosci 5(2): 146156. Sippl, C. and Tamm, E. R. (2014). "What is the nature of the RGC-5 cell line?" Adv Exp Med Biol 801: 145-154. Song, Y., Chen, X., Wang, L. Y., Gao, W. and Zhu, M. J. (2013). "Rho kinase inhibitor fasudil protects against beta-amyloid-induced hippocampal neurodegeneration in rats." CNS Neurosci Ther 19(8): 603-610. Stroman, G. A., Stewart, W. C., Golnik, K. C., Cure, J. K. and Olinger, R. E. (1995). "Magnetic resonance imaging in patients with low-tension glaucoma." Arch Ophthalmol 113(2): 168-172. Sugiyama, T., Shibata, M., Kajiura, S., Okuno, T., Tonari, M., Oku, H. and Ikeda, T. (2011). "Effects of fasudil, a Rho-associated protein kinase inhibitor, on optic nerve head blood flow in rabbits." Invest Ophthalmol Vis Sci 52(1): 64-69. Sumi, T., Matsumoto, K. and Nakamura, T. (2001). "Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase." J Biol Chem 276(1): 670-676. Sung, J. K., Miao, L., Calvert, J. W., Huang, L., Louis Harkey, H. and Zhang, J. H. (2003). "A possible role of RhoA/Rho-kinase in experimental spinal cord injury in rat." Brain Res 959(1): 29-38. Suzuki, Y., Shibuya, M., Satoh, S.-i., Sugimoto, Y. and Takakura, K. (2007). "A postmarketing surveillance study of fasudil treatment after aneurysmal subarachnoid hemorrhage." Surg Neurol 68(2): 126-131. Tanihara, H., Inatani, M., Honjo, M., Tokushige, H., Azuma, J. and Araie, M. (2008). "Intraocular pressure-lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers." Arch Ophthalmol 126(3): 309-315. Thanos, S., Naskar, R. and Heiduschka, P. (1997). "Regenerating ganglion cell axons in the adult rat establish retinofugal topography and restore visual function." Exp Brain Res 114(3): 483-491. Toft-Kehler, A. K., Skytt, D. M., Poulsen, K. A., Braendstrup, C. T., Gegelashvili, G., Waagepetersen, H. and Kolko, M. (2014). "Limited energy supply in Muller cells alters glutamate uptake." Neurochem Res 39(5): 941-949. 47 Tokushige, H., Inatani, M., Nemoto, S., Sakaki, H., Katayama, K., Uehata, M. and Tanihara, H. (2007). "Effects of topical administration of y-39983, a selective rho-associated protein kinase inhibitor, on ocular tissues in rabbits and monkeys." Invest Ophthalmol Vis Sci 48(7): 32163222. Tokushige, H., Waki, M., Takayama, Y. and Tanihara, H. (2011). "Effects of Y-39983, a selective Rhoassociated protein kinase inhibitor, on blood flow in optic nerve head in rabbits and axonal regeneration of retinal ganglion cells in rats." Curr Eye Res 36(10): 964-970. Tonges, L., Frank, T., Tatenhorst, L., Saal, K. A., Koch, J. C., Szego, E. M., Bahr, M., Weishaupt, J. H. and Lingor, P. (2012). "Inhibition of rho kinase enhances survival of dopaminergic neurons and attenuates axonal loss in a mouse model of Parkinson's disease." Brain 135(Pt 11): 33553370. Tonges, L., Gunther, R., Suhr, M., Jansen, J., Balck, A., Saal, K. A., Barski, E., Nientied, T., Gotz, A. A., Koch, J. C., Mueller, B. K., Weishaupt, J. H., Sereda, M. W., Hanisch, U. K., Bahr, M. and Lingor, P. (2014). "Rho kinase inhibition modulates microglia activation and improves survival in a model of amyotrophic lateral sclerosis." Glia 62(2): 217-232. Tonges, L., Koch, J. C., Bahr, M. and Lingor, P. (2011). "ROCKing Regeneration: Rho Kinase Inhibition as Molecular Target for Neurorestoration." Front Mol Neurosci 4: 39. Toshima, Y., Satoh, S., Ikegaki, I. and Asano, T. (2000). "A new model of cerebral microthrombosis in rats and the neuroprotective effect of a Rho-kinase inhibitor." Stroke 31(9): 2245-2250. Tsukita, S. and Yonemura, S. (1997). "ERM proteins: head-to-tail regulation of actin-plasma membrane interaction." Trends Biochem Sci 22(2): 53-58. Tura, A., Schuettauf, F., Monnier, P. P., Bartz-Schmidt, K. U. and Henke-Fahle, S. (2009). "Efficacy of Rho-kinase inhibition in promoting cell survival and reducing reactive gliosis in the rodent retina." Invest Ophthalmol Vis Sci 50(1): 452-461. Uehata, M., Ishizaki, T., Satoh, H., Ono, T., Kawahara, T., Morishita, T., Tamakawa, H., Yamagami, K., Inui, J., Maekawa, M. and Narumiya, S. (1997). "Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension." Nature 389(6654): 990-994. Van de Velde, S., Van Bergen, T., Sijnave, D., Hollanders, K., Castermans, K., Defert, O., Leysen, D., Vandewalle, E., Moons, L. and Stalmans, I. (2014b). "AMA0076, a novel, locally acting Rho kinase inhibitor, potently lowers intraocular pressure in New Zealand white rabbits with minimal hyperemia." Invest Ophthalmol Vis Sci 55(2): 1006-1016. Van Veldhuisen, P. C., Ederer, F., Gaasterland, D. E., Sullivan, K., Beck, A., Prum, B. E., Cyrlin, M. N. and Weiss, H. (2000). "The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. ." Am J Ophthalmol 130(4): 429-440. Villar-Cheda, B., Dominguez-Meijide, A., Joglar, B., Rodriguez-Perez, A. I., Guerra, M. J. and Labandeira-Garcia, J. L. (2012). "Involvement of microglial RhoA/Rho-kinase pathway activation in the dopaminergic neuron death. Role of angiotensin via angiotensin type 1 receptors." Neurobiol Dis 47(2): 268-279. Waki, M., Yoshida, Y., Oka, T. and Azuma, M. (2001). "Reduction of intraocular pressure by topical administration of an inhibitor of the Rho-associated protein kinase." Curr Eye Res 22(6): 470474. Walker, K. L., Yoo, H. K., Undamatla, J. and Szaro, B. G. (2001). "Loss of neurofilaments alters axonal growth dynamics." J Neurosci 21(24): 9655-9666. Wang, J. J., Mitchell, P. and Smith, W. (1997). "Is there an association between migraine headache and open-angle glaucoma? Findings from the Blue Mountains Eye Study." Ophthalmology 104(10): 1714-1719. Weibel, D., Kreutzberg, G. W. and Schwab, M. E. (1995). "Brain-derived neurotrophic factor (BDNF) prevents lesion-induced axonal die-back in young rat optic nerve." Brain Res 679(2): 249-254. Weinreb, R. N. (2007). "Glaucoma neuroprotection: What is it? Why is it needed?" Can J Ophthalmol 42(3): 396-398. 48 Weinreb, R. N. and Khaw, P. T. (2004). "Primary open-angle glaucoma." Lancet 363(9422): 17111720. Weise, J., Isenmann, S., Klocker, N., Kugler, S., Hirsch, S., Gravel, C. and Bahr, M. (2000). "Adenovirusmediated expression of ciliary neurotrophic factor (CNTF) rescues axotomized rat retinal ganglion cells but does not support axonal regeneration in vivo." Neurobiol Dis 7(3): 212-223. Wiederholt, M., Thieme, H. and Stumpff, F. (2000). "The regulation of trabecular meshwork and ciliary muscle contractility." Prog Retin Eye Res 19(3): 271-295. Wierzbowska, J., Robaszkiewicz, J., Figurska, M. and Stankiewicz, A. (2010). "Future possibilities in glaucoma therapy." Med Sci Monit 16(11): RA252-259. Wolfs, R. C., Klaver, C. C., Ramrattan, R. S., van Duijn, C. M., Hofman, A. and de Jong, P. T. (1998). "Genetic risk of primary open-angle glaucoma. Population-based familial aggregation study." Arch Ophthalmol 116(12): 1640-1645. Yamamoto, K., Maruyama, K., Himori, N., Omodaka, K., Yokoyama, Y., Shiga, Y., Morin, R. and Nakazawa, T. (2014). "The novel Rho kinase (ROCK) inhibitor K-115: a new candidate drug for neuroprotective treatment in glaucoma." Invest Ophthalmol Vis Sci. Yamashita, T. and Tohyama, M. (2003). "The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI." Nat Neurosci 6(5): 461-467. Yan, D. B., Coloma, F. M., Metheetrairut, A., Trope, G. E., Heathcote, J. G. and Ethier, C. R. (1994). "Deformation of the lamina cribrosa by elevated intraocular pressure." Br J Ophthalmol 78(8): 643-648. Yanagi, M., Kawasaki, R., Wang, J. J., Wong, T. Y., Crowston, J. and Kiuchi, Y. (2011). "Vascular risk factors in glaucoma: a review." Clin Experiment Ophthalmol 39(3): 252-258. Zhang, C. W., Lu, Q., You, S. W., Zhi, Y., Yip, H. K., Wu, W., So, K. F. and Cui, Q. (2005). "CNTF and BDNF have similar effects on retinal ganglion cell survival but differential effects on nitric oxide synthase expression soon after optic nerve injury." Invest Ophthalmol Vis Sci 46(4): 1497-1503. Zhang, Z., Ottens, A. K., Larner, S. F., Kobeissy, F. H., Williams, M. L., Hayes, R. L. and Wang, K. K. (2006). "Direct Rho-associated kinase inhibition [correction of inhibiton] induces cofilin dephosphorylation and neurite outgrowth in PC-12 cells." Cell Mol Biol Lett 11(1): 12-29. Zhao, J., Zhou, D., Guo, J., Ren, Z., Zhou, L., Wang, S., Xu, B. and Wang, R. (2006). "Effect of Fasudil Hydrochloride, a Protein Kinase Inhibitor, on Cerebral Vasospasm and Delayed Cerebral Ischemic Symptoms After Aneurysmal Subarachnoid Hemorrhage." Neurol Med Chir 46(9): 421-428. Zhou, Y., Huang, X., Hecker, L., Kurundkar, D., Kurundkar, A., Liu, H., Jin, T. H., Desai, L., Bernard, K. and Thannickal, V. J. (2013). "Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis." J Clin Invest 123(3): 1096-1108. 49