What*s in my vanilla

What’s in my vanilla? Practice exercise. (10 points)
1. (2 points) Complete table 1.
#p (.25)
#n (.75) exact mass (.25) + sig figs (.25)
% abund (.25) + sig figs (.25)
Table 1
# of protons #of neutrons Exact mass % abundance
20
Ne
10 10
19.9924356
90.48
21 Ne
10 11
20.9938428
0.27
22 Ne
10 12
21.9913831
9.25
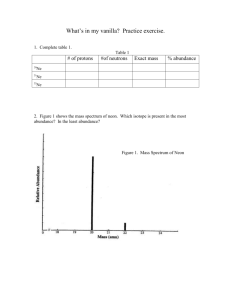
2. (1 point) Figure 1 shows the mass spectrum of neon. Which isotope is present in the most abundance? In the least abundance?
Figure 1. Mass Spectrum of Neon
3. (3 points total) The mass spectrum of mercury is shown in figure 2. Use a ruler to help you complete table 2. Be sure to use correct sig figs. when doing addition, division, etc.
-Measured to nearest 0.1 mm (.25)
-Added up all measurements, divided to get % of each (.5) + sf (.25)
-Multiplied each by fractional %, added to get experimental MM (.5) + sf (.25)
-% Error (.5)
Most abundant (.5)
Closest (.25)
Figure 2. Mass Spectrum of Mercury.
Isotope
Table 2.
Mass (amu) Measurement (mm) Isotopic Abundance (%)
198 Hg + 198 19.5 19.5/195.8 = 9.96
199
200
201
202
204
32.5
45.8
25.9
59.0
13.1
16.6
23.4
13.2
30.1
6.67
*Experimental molar mass of mercury:19.7(2)+ 33.0(3) + 46.8(0) + 26.5(3) + 60.8(0) +
13.6(0) = 200.5
Accepted molar mass of mercury:200.59
*Percent error in molar mass: (200.59-200.5)/200.59 X 100 = 0.04%
Most abundant isotope of mercury: 202 Hg
Isotope closest to the molar mass: 201 Hg
*Sample calculation (include one example of % isotopic abundance; MM
Hg
; % error)
4. (3 points total) The mass spectrum of elemental bromine (Br
2
) is shown in figure 3.
Use a ruler to help you complete table 3. Be sure to use correct sig figs.
-Identify peaks for Br
2
as well as Br (.75)
-Measured to nearest 0.1 mm (.25)
-Added up all measurements, divided to get % of each (.5) + sf (.25)
-Multiplied each by fractional %, added to get experimental MM (.5) + sf (.25)
-% Error (.5)
Figure 3. Mass spectrum of Br
2
Mass (amu)
79
Table 3.
Species
79 Br +
Measurement (mm)
38.5 (49.7%)
81 81 Br + 38.9 (50.3%)
Total = 77.4
*Experimental molar mass of Br: .497*79 + .503*81 = 80.0
Accepted molar mass of Br: 79.904
*Percent error in molar mass 0.1%
*Sample calculations:
5. (3 points total)
A. (1 point) What is the molecular mass of vanillin containing all carbon-12? What is the molecular mass of vanillin containing one carbon-13?
12 C vanillin = 152
13
C vanillin = 153
B. (1 point) Describe what you would expect the peaks in the mass spectrum of vanillin to look like.
Since
12
C has a much greater abundance, there should be a large peak for the 152 and a small peak at 153.
C. (1 point) Would you expect to see a peak in the mass spectrum for vanillin containing two carbon-13 atoms? Why or why not?
No the abundance of 13 C is 1% so, the peak for a compound having 2 13 C atoms would be
1% of 1% (actually 8% of 8%) so it would be too small to see.
6. Your lab group should have received packets of GC/MS data containing chromatograms and accompanying mass spectra for five different samples:
1) Real vanilla extract
2) Imitation vanilla extract
3) Vanilla bean
4) Real vanilla extract contaminated with Coumarin.
Use these data packets to answer the following questions.
A. (1 point) What is the difference between a chromatogram and a mass spectrum?
The chromatogram represents total abundance vs. time. While a mass spectrum shows abundance vs m/z. Each peak in the chromatogram is a separate compound, while each peak in a mass spectrum represents fragments of one specific compound (peak from the chromatogram.)
B. (1 points) Why is there only one chromatogram but multiple mass spectra in each packet?
Each peak in the chromatogram has its own mass spectrum.
C. (1 point) What does each peak in the chromatogram represent? What does each peak in the mass spectrum represent? Each peak in the chromatogram represents a compound. Each peak in the mass spectrum represents abundance of a certain mass of ion.
D. (1 point) The main flavor component in vanilla is vanillin, C
3
H
8
O
3
. What is the retention time for the vanillin? How did you identify it? Vanillin comes at 11.7 minutes. It was identified because its mass spectrum has a peak at 152. ( not because it was the biggest peak in the chromatogram)
E. (1 point) If the molecular mass of vanillin is 152 amu, why is there a large 151 peak in the mass spectrum of vanillin? What is the 153 peak? The 151 peak represents (M-H)
+ peak. The 153 peak represents the 13 C isotope peak.
F. (1 point) Is the vanillin present in all of the samples? What does this mean about the main flavor component in imitation compared to real vanilla?
Vanillin is present in all of the samples because it is the main flavor component.
Imitation vanilla has the same main flavor compent – thus imitation and real taste similar
(although not identical – see G)
G. (1 point) If the molecular formula of coumarin is C
9
H
6
O
2
, how can you identify the coumarin contaminant in the real vanilla extract? Coumarin has a MM of 146. The peak at 11.4 minutes in the spectrum of the tainted vanilla has a peak at 11.4 minutes that has a M
+
peak at 146.
H. (1 point) Are there any differences between the real vanilla extract, the imitation vanilla extract, and the vanilla bean? What are they and what do you think they represent? Would these differences be reflected in any flavor differences?
The chromatogram of the vanilla bean shows multiple smaller peaks in addition to the vanillin peak. These could represent other compounds in the bean, that could produce a subtly different taste. The real vanilla and imitation vanilla generally have the same main peak; there are a few other peaks that could be from preservatives and additives in the extract.