AIC_12562_sm_suppinfo
advertisement

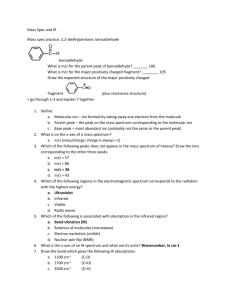
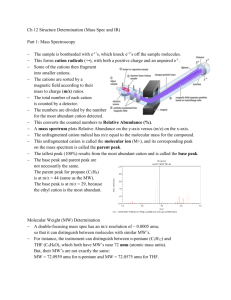
Supporting Information 1 2 3 Contents: Figure S1. XPS C1s spectrum of the MWCNT. Figure S2. XPS C1s 4 5 spectrum of the functionalized MWCNT. Figure S3. XPS N1s spectrum of the functionalized MWCNT. 6 7 The C 1s spectrum of the MWCNT is displayed in Figure S1. This spectrum clearly 8 consists of four components. The peak at 284.9 eV is is due to the graphite-like 9 carbon atoms of the tube walls.1,2 The peak at 285.8 is due to defect-containing sp2- 10 hybridized carbon. The peak at 288.9 eV originates from carboxylic groups. 3 The 11 peaks at 291.6 eV is assigned to sp2-hybridized carbon *←shake-up.1,2 Figure S2 12 shows the C1s spectrum of MWCNT/p-chitosan. The peak at 285.0 eV is contributed 13 by graphite-liked carbon. The peak at 286.7 eV is assigned to –C=O, –C–NH2, and – 14 C-O-.1 The peak at 288.5 eV is due to –CO–NH–. The peak at 289.7 eV originates 15 from carboxylic groups. The N1s spectrum of functionalized MWCNT is presented in 16 Figure S3 .The peak at 399.7 eV is assigned to the –C–NH2 . 1 The peak at 400.7 eV is 17 due to the –CO–NH–. It is obvious that the nitrogen-containing groups are from p- 18 chitosan. 19 20 Literature cited 21 1. Liu Y, Yu ZL, ZhangYM, Guo DS, Liu YP. Supramolecular Architectures of - 22 Cyclodextrin-Modified Chitosan and Pyrene Derivatives Mediated by Carbon 23 Nanotubes and Their DNA Condensation J. Am. Chem. Soc. 2008; 130: 10431- 24 10439. 25 2 Holzinger M, Abraham J, Whelan P, Graupner R, Ley L, Hennrich F et al. 26 Functionalization of Single-Walled Carbon Nanotubes with (R-)Oxycarbonyl 27 Nitrenes. J. Am. Chem. Soc. 2003; 125: 8566-8580 28 3 Urszula DW, Viera S, Ralf G, Sung HJ, Byung HK, Hyun JL. Effect of SOCl2 29 Treatment on Electrical and Mechanical Properties of Single-Wall Carbon 30 Nanotube Networks. J. Am. Chem. Soc. 2005; 127: 5125-5131 31