Atomic Radius: Trends in the Periodic Table
advertisement

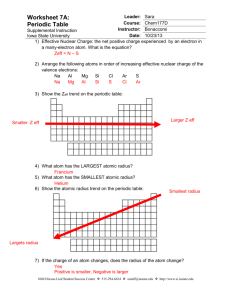
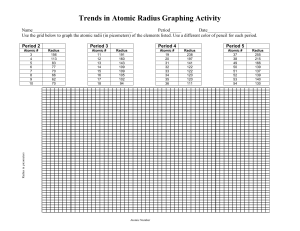
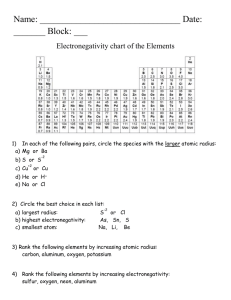
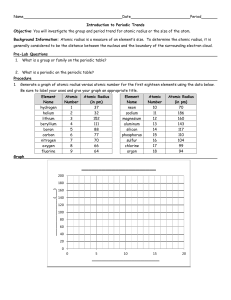
Atomic Radius: Trends in the Periodic Table Use two different colored pencils to indicate metals, nonmetals, and metalloids on the blank periodic table below. Allow the two colors to blend for the metalloids. Provide a key for identification. Label each of the family names for the groups, include the lanthanide and actinide series. Then draw arrows that indicate increasing atomic radius. Introduction to Chemistry Page 1 Atomic Radius: Trends in the Periodic Table Analysis: 1. Which of the following atoms has the largest atomic radius? a. Li b. Xe c. Cl d. Cs 2. Which of the following atoms has the smallest atomic radius? a. K b. Cs c. Pb d. Cl 3. An atom of which member of the Carbon family is the smallest? 4. An atomic of which member of the Halogen family is the largest? 5. Which atom has the smallest atomic radii? Draw a Bohn Model of this atom. Explain why it has the smallest radius? 6. Which atom has the largest atomic radii? Draw a Bohr Model of this atom. Explain why it has the largest radius? Introduction to Chemistry Page 2