Chemistry Unit 8 Guided Notes
advertisement

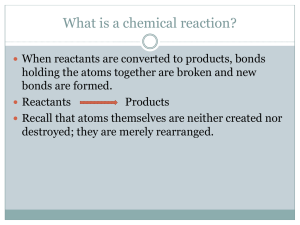
How Can We Describe Chemical Reactions? Chemistry Unit 8: Guided Notes New Skills Identify different types of reactions Balance chemical reactions Predict solubility and replacement reactions Write net-ionic equations Academic Language: Aqueous solution Chemical equation Chemical reaction Coefficient Combustion reaction Complete ionic equation Decomposition reaction Double-replacement reaction Insoluble Net ionic equation Precipitate Product Reactant Single-replacement reaction Soluble Solute Solvent Spectator ion Synthesis reaction 1 Unit 8 Homework: CALM: http://calm.indiana.edu/ 8.1 Reactions and Equations 10 questions 8.2 Classifying Chemical Reactions 10 questions 8.3 Solubility 10 questions 8.4 Reactions in aqueous solutions 10 questions 8.5 Accumulating Content and Skills 10 questions 2 Chemistry Unit 8: Learning Goals and Objectives 8.1 Reactions and Equations – Chemical reactions are represented by balanced chemical equations o Recognize evidence of chemical change. o Represent chemical reactions with equations. o Balance chemical equations 8.2 Classifying Chemical Reactions – There are four types of chemical reactions: synthesis, combustion, decomposition, and replacement reactions. o Classify chemical reactions o Identify the characteristics of different classes of chemical reactions 8.3 Solubility - When solutions containing ionic substances are mixed, ions interchange and can form solids. o Identify new possible ionic compounds in a reaction o Define the terms soluble and insoluble o Predict solids based on solubility rules 8.4 Reactions in aqueous solutions - Double –replacement reactions occur between substances in aqueous solutions and produce precipitates, water, or gases. o Describe aqueous solutions o Write complete ionic and net ionic equations for chemical reactions in aqueous solutions. o Predict whether reactions in aqueous solutions will produce a precipitate, water, or a gas. 8.5 Accumulating Content and Skills:– Chemistry content is continuous and builds on prior knowledge and skills. This section will combine this unit with previous units. o Apply knowledge and skills from previous units to content learned in this unit. 3 8.1 Reactions and Equations – Chemical reactions are represented by balanced chemical equations Chemical Reactions Objective: Recognize evidence of chemical change. Chemical reaction- a process by which the atoms of one or more substances are rearranged to form different substances. Evidence of a chemical reaction is a … Evidence: Representing Chemical Reactions Objective: Represent chemical reactions with equations. Chemical equations – statements that show chemical reactions by the use of chemical formulas and conserved matter with the relative amounts of substances in the reaction. Parts of an equation reaction: Reactants – Products – Common symbols 4 Representing Reactions Word equation – use of words for reactants and products Skeleton equation – chemical formulas used for reactants and products but not balanced. Chemical Equation - Is a statement that uses chemical formulas to show the identities and relative amounts of the substances involved in a chemical reaction. Balancing Chemical Reactions Objective: Balance chemical equations Coefficient - in a chemical equation it is the number written in front of a reactant or product, describing the lowest whole-number ratio of the amounts of all the reactants and products. 5 Steps for balancing equations: 1. Write the skeleton equation: a. Make sure chemical formulas are correct b. Put in symbols and physical states liquid sodium carbonate + aqueous calcium chloride yields solid calcium carbonate + aqueous sodium chloride 2. Count the atoms of the elements in the reactants a. Group intact polyatomic ions as a single substance Na2CO3(l) + CaCl2(aq) CaCO3(s) + NaCl(aq) Sodium: Carbonate: Calcium: Chloride: 3. Count the atoms of the elements in the products a. Group intact polyatomic ions as a single substance Na2CO3(l) + CaCl2(aq) CaCO3(s) + NaCl(aq) Sodium: 2 Sodium: Carbonate: 1 Carbonate: Calcium: 1 Calcium: Chloride: 2 Chloride: 6 4. Change the coefficients to make the number of atoms of each element equal on both sides of the equation a. Never change subscripts Na2CO3(l) + CaCl2(aq) CaCO3(s) + NaCl(aq) 5. Write the coefficients in their lowest possible ratios Na2CO3(l) + CaCl2(aq) CaCO3(s) + 2NaCl(aq) 6. Go back and check math Example: aqueous sodium hydroxide + aqueous calcium bromide yields solid calcium hydroxide and aqueous sodium bromide ***practice problems p287 #4-6*** ***p288 #7-13** 7 8.2 Classifying Chemical Reactions – There are four types of chemical reactions: synthesis, combustion, decomposition, and replacement reactions. Some reactions can fit more than one reaction type. Types of Chemical Reactions Objective: Classify chemical reactions Objective: Identify the characteristics of different classes of chemical reactions Chemists classify reactions in order to organize the many types. Synthesis – a chemical reaction in which two or more substances react to produce a single product. When two compounds react o A + B AB; when two elements react. o AB + CD ABCD; AB + BC ABC Combustion – oxygen combines with a substance and releases energy in the form of heat and light. Example: o Element and oxygen react: A + O2 AO o Compound and oxygen react: AB + O2 AO + B 8 Decomposition – a single compound breaks down into two or more elements or new compounds o Compound breaks down into two elements: AB A + B o Compound breaks down to form new compounds: ABCD AC + BD Replacement / Displacement Single Displacement – the atoms of one element replace the atoms of another element in a compound. o A + BX B + AX Activity Series o A metal will not always replace another metal in a compound dissolved in water because of differing reactivities. - some metals are more reactive than others o Halogens frequently replace other halogens in replacement reactions. Metals/Halogens are listed in order of reactivity. A less reactive metal/halogen will not replace a more reactive metal/halogen. ***p 291 #14-17; page 292 #18-20, p295 #21-24*** 9 Double replacement – also called metathesis - the exchange of ions between two compounds. o AX + BY AY + BX; A and B are cations, XY are anions Metathesis reactions often forms one of three products: o Precipitate o Water o Gas 10 ***p297 #25-28*** ***p298 #29-34*** 11 8.3 Solubility - When solutions containing ionic substances are mixed, ions interchange and can form solids. Objective: Identify new possible ionic compounds in a reaction Objective: Define the terms soluble and insoluble Ionic Compounds in Solutions Ionic compounds in aqueous solutions mix and exchange partners (double replacement). example: Na2SO4 + CaCl2 Solubility Objective: Predict solids based on solubility rules Solubility rules are used to determine the state of matter of products in an aqueous solution. Soluble Insoluble Solubility Rules 1. Most nitrate (NO3) salts are soluble 2. Most salts containing the alkali metal ions (Li+, Na+, K+, Cs+, Rb+) and the ammonium ion (NH4+) are soluble. 3. Most chloride, bromide, and iodide salts are soluble a. Exceptions: Ag+, Pb2+, Hg22+ 4. Most sulfate salts are soluble . a. Exceptions: Bas+, Pb2+, Hg22+, and Ca2+ 5. Most hydroxides are only slightly soluble (treat as insoluble). a. Exceptions: Na+, K+ 6. Most sulfide (S2-), carbonate (CO32-), chromate (CrO42-), and phosphate (PO43-) salts are only slightly soluble (treat as insoluble). a. Exceptions: any containing Alkali metals and ammonium. 12 Solubility Summary: Rule Soluble Insoluble #1 #2 #3 #4 #5 #6 Nitrates (NO3-) Group 1, NH4+ Halogens Sulfates (SO42-) No exceptions No exceptions Ag+, Pb2+, Hg22+ Bas+, Pb2+, Hg22+, Ca2+ Hydroxides (OH-) S2-, CO32-, CrO42- PO43- Example Problem: Aqueous silver nitrate mixes with aqueous sodium chloride; what solid will be produced from this solution. 13 8.4 Reactions in aqueous solutions - Double –replacement reactions occur between substances in aqueous solutions and produce precipitates, water, or gases. Objective: Describe aqueous solutions Aqueous solution – contains one or more dissolved substances (called solutes) in water. Solution – Solutes – Solvent – There are many possible solutes—sugar and alcohol are molecular compounds that exist as molecules in aqueous solutions. Objective: Write complete ionic and net ionic equations for chemical reactions in aqueous solutions. Types of Aqueous Equations Ionic Equation: equations that show ionic detail and dissociation within reactions. Formula Equationo 2NaOH(aq) + CuCl2(aq) 2NaCl(aq) + Cu(OH)2(s) Complete ionic equationo 2Na+(aq) + 2OH-(aq) + Cu2+(aq) + 2Cl-(aq) 2Na+(aq) + 2Cl-(aq) + Cu(OH)2(s) o Spectator Ions- 14 Net ionic equation – o 2OH-(aq) + Cu2+(aq) Cu(OH)2(s) Some reactions produce more water molecules. HBr(aq) + NaOH(aq) → H2O(l) + NaBr(aq) Without spectator ions : Gases that are commonly produced are carbon dioxide, hydrogen cyanide, and hydrogen sulfide. 2HI(aq) + Li2S(aq) → H2S(g) + 2LiI(aq) HCl(aq) + NaHCO3(aq) → H2CO3(aq) + NaCl(aq) H2CO3(aq) decomposes immediately. H2CO3(aq) → H2O(l) + CO2(g) Two reactions can be combined and represented by a single chemical reaction (wait, don’t write down anything from the picture) Reaction 1: HCl(aq) + NaHCO3(aq) → H2CO3(aq) + NaCl(aq) Reaction 2: H2CO3(aq) → H2O(l) + CO2(g) Combined Equation: HCl(aq) + NaHCO3(aq) + H2CO3(aq) → H2CO3(aq) + NaCl(aq) + H2O(l) + CO2(g) Overall Equation: 15 Objective: Predict whether reactions in aqueous solutions will produce a precipitate, water, or a gas. Types of Reactions in Aqueous Solutions: Double replacement 1. Precipitate is formed- when a compound forms from ions, an exothermic reaction takes place. The ions by themselves are less stable and therefore of higher energy than when combined in a compound. This change in energy (increase in stability) ‘drives’ the reaction. Example: 2NaOH(aq) + CuCl2(aq) 2NaCl(aq) + Cu(OH)2(s) Net: 2. Water is formed- even though a double reaction takes place, solutions may look the same since water will still be the dominant substance. Example: HBr(aq) + NaOH(aq) H2O(l) + NaBr(aq) Net: 3. Gas is formed- for the same reasons that a precipitate is formed, a gas will also form. Common gases in double replacement reactions are: carbon dioxide, hydrogen cyanide, and hydrogen sulfide. Example: 2HI(aq) + Li2S(aq) H2S(g) + 2LiI(aq) Net: ***practice problems p302 #35-39*** ***p304 #40-44, p306 #45-49, p308 #50-56*** 16 8.5 Accumulating Content and Skills:– Chemistry content is continuous and builds on prior knowledge and skills. This section will combine this unit with previous units. Objective: Apply knowledge and skills from previous units to content learned in this unit. Why do polyatomic ions stay intact in an aqueous solution? How do naming rules change when working with gases vs. aqueous acids? How does lattice energy relate to solubility rules? Key Concepts Some physical changes are evidence that indicate a chemical reaction has occurred. Word equations and skeleton equations provide important information about a chemical reaction. A chemical equation gives the identities and relative amounts of the reactants and products that are involved in a chemical reaction. Balancing an equation involves adjusting the coefficients until the number of atoms of each element is equal on both sides of the equation. 17 Classifying chemical reactions makes them easier to understand, remember, and recognize. Activity series of metals and halogens can be used to predict if singlereplacement reactions will occur. In aqueous solutions, the solvent is always water. There are many possible solutes. Many molecular compounds form ions when they dissolve in water. When some ionic compounds dissolve in water, their ions separate. When two aqueous solutions that contain ions as solutes are combined, the ions might react with one another. The solvent molecules do not usually react. Reactions that occur in aqueous solutions are double-replacement reactions. 18