Sci 2014 NKAL test 2 - Fort Thomas Independent Schools
advertisement

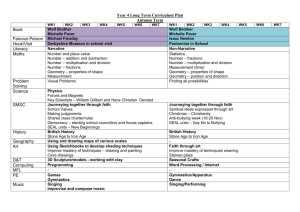
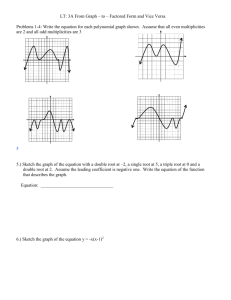
Science Exam #2 2014-15 – High School - Practice Questions Student Instructions: Write your complete ID code on your answer sheet. For each question, choose the BEST answer. If you change your answer, erase well. 1. The troponin/tropomyosin complex regulates the crossbridge binding of myosin to actin in the contractile unit of a muscle fiber. What ion is necessary for the conformational change in troponin/tropomyosin complex to allow for muscle contraction? A. Ca2+ B. PO43C. K+ D. Na+ 2. Blood must be able to efficiently carry oxygen to the tissues and waste carbon dioxide away from the tissues. What phenomenon occurs when the deoxygenation of blood increases its ability to carry carbon dioxide? A. perfusion B. chloride shift C. Haldane effect D. Bohr effect 3. Xylem and phloem are vascular tissues in plants. What group of plants does NOT possess vascular tissue? A. angiosperms B. bryophytes C. pteridophytes D. gymnosperms 4. High root pressures will force some water to exit the leaves without transpiration occurring. What is the exudation of water from leaves due to excess root pressure? A. dew formation B. sapping C. tapping D. guttation 5. Some plants like the pineapple are adapted to arid conditions by possessing alternative carbon fixation pathways that store carbon dioxide overnight as a type of organic acid. In what substance do these plants store carbon from nighttime until the next day, when it can be used during photosynthesis? A. malate B. pyruvate C. ribulose bisphosphate D. rubisco 6. Also called collar cells, these cells possess a flagellum that is used to generate mild water currents to aid in filter feeding. What are these specialized flagellated cells that line the interior of sponge cavities as seen on the right? A. choanocyte B. gemmule C. nematocyst D. cnidocyte 2014-15-– Science –High School – Practice Questions - Page 1 7. Also known as a vomeronasal organ, this olfactory organ is found in animals such as some reptiles. What auxiliary sense organ is responsible for the heightened sense of smell in snakes that allows them to detect prey? A. paratoid gland B. Jacobson’s organ C. Flehmen organ D. salt gland 8. During DNA replication, the double helix must be opened to allow for the synthesis of a new complementary strand. Which enzyme “unzips” DNA during replication? A. topoisomerase B. ligase C. helicase D. polymerase 9. Consider an organism with the genotype AaBbcc that is crossed with an organism with genotype AabbCc. Assuming all inheritance is complete and neglecting crossing over, what is the probability that this mating will produce at least one offspring that is purely recessive (aabbcc)? A. 1/16 B. 1/8 C. 1/2 D. 9/16 10. Match the cellular structure with its function: x. smooth endoplasmic reticulum y. lysosome z. Golgi apparatus A. B. C. D. 1. modification, packaging, and secretion of cellular products 2. the hydrolytic breakdown of organic substances 3. the biosynthesis and transport of lipids x-1, y-2, z-3 x-2, y-1, z-3 x-3, y-1, z-2 x-3, y-2, z-1 11. Facilitated diffusion is a means of speeding up general diffusion by the use of a variety of intramembrane proteins. Which of the following substances is most likely to require facilitated diffusion in order to enter/exit the cell in a timely manner? A. O2 B. glucose C. cholesterol D. CO2 12. Crossing over is a phenomenon in which homolog chromosomes exchange genes along the length of the chromosome. What is the point at which crossing over occurs, as seen in the diagram to the right? A. centromere B. chiasma C. kinetochore D. telomere 2014-15-– Science –High School – Practice Questions - Page 2 13. These unicellular amoeboid protists possess a calcareous test and are found primarily in the ocean. What group of organisms leaves behind the calcium carbonate “shells” that can provide the sediment that forms many limestones? A. radiolaria B. cyanobacteria C. foraminifera D. diatoms 14. This group of fungi is the largest phylum, containing the morels, cup fungi, and ergot. What fungi group is called the “sac fungi” due to their microscopic reproductive structures? A. Ascomycota B. Basidiomycota C. Deuteromycota D. Zygomycota 15. Invented in the 1950s by scientists in the Soviet Union, this reactor is a magnetic field confinement device that contains plasma into a well-defined torus shape. What is this type of fusion reactor that may be the leading method of developing useful fusion energy in the future? A. stellarator B. breeder reactor C. heliotron D. tokamak 16. Located in Niagara Falls, New York, this residential neighborhood was the site of a 21,000 tons toxic waste burial site. What environmental disaster was called a “national symbol of a failure to exercise a sense of concern for future generations” by then New York Health Department Commissioner David Axelrod? A. Three Mile Island B. Bhopal C. Love Canal D. Cuyahoga 17. This term can be applied to animals such as the Pacific salmon and mayfly insects. What term refers to any animal that produces huge numbers of offspring in one reproductive event during its life cycle? A. autogamous B. iteroparous C. isogamous D. semelparous 18. Chemical reactions in chemical equilibrium are capable of reverse reactions. Which statement is true concerning a reaction at chemical equilibrium? A. For any reaction at chemical equilibrium, entropy continues to increase. B. For any reaction at chemical equilibrium, the rate of product formation will be greater than the rate of the reversible reaction. C. For any reaction at chemical equilibrium, the Gibbs free energy will be zero. D. For any reaction at chemical equilibrium, the activation energy cannot be overcome. 19. Consider the reaction AgNO3 (aq) + KCl (aq) AgCl (s) + KNO3 (aq). In this reaction AgCl is a solid that forms during a double displacement reaction that then falls out of solution due to gravimetric settling. What is this solid substance called? A. pigment B. precipitate C. salt D. supernate 2014-15-– Science –High School – Practice Questions - Page 3 20. Match the element with its electronegativity value: x. Fluorine y. Chlorine z. Oxygen A. B. C. D. 1. 4.0 2. 3.0 3. 3.5 x-1, y-2, z-3 x-1, y-3, z-2 x-2, y-1, z-3 x-2, y-3, z-1 21. This guideline describes the electron configuration and the filling of atomic orbitals. What does the figure to the right show? A. Pauli exclusion principle B. periodic blocking C. Hund’s rule D. Klechkowski’s rule 22. Gibbs Free Energy (G) is essentially the energy that is available to do work in a chemical reaction and the change in G has a profound effect on the direction and likelihood of a reaction. Which statement is true for the equation ΔG < 0? A. The chemical reaction is nonspontaneous. B. The chemical reaction is spontaneous in the forward direction. C. The system is at equilibrium. D. The entropy of the system will decrease. 23. Consider 1 L of an ideal gas at a temperature of 300 K and 1 atm. Calculate the new gas volume when it is heated to 400 K and under a pressure of 2 atm. A. 0.67 L B. 1L C. 1.3 L D. 2.6 L 24. Nitric acid is considered to be a strong acid due to its ability to ionize completely in water. What is the conjugate base of nitric acid? A. NO2B. H2(NO3)+ C. H+ D. NO3- 25. Assume the molar mass of sulfuric acid (H2SO4) to be 98 g in this problem. What is the molality (m) of a solution in which 10 g of H2SO4 is dissolved in 100 g of water? A. 0.00102 m B. 0.102 m C. 1.02 m D. 10.2 m 2014-15-– Science –High School – Practice Questions - Page 4 26. Fractional crystallization is a process used to isolate and purify substances. From the information presented in the graph to the right, which compound will fall out of solution completely first when that solution is cooled to 0°C? A. KCl B. NaCl C. Neither. The solution will freeze at 0°C, trapping each compound in the icy matrix. D. Each will fall out of solution at the same time, because both are chlorine salts. 27. This form of radiation results when a charged particle passes through a material at a speed greater than the phase velocity of light in that medium. What radiation of nuclear reactors exhibits a characteristic blue glow? A. synchrotron light B. Bremsstrahlung radiation C. Askaryan effect D. Cherenkov radiation 28. Consider the decay of a carbon-10 atom into a boron-10 atom: describes this reaction? A. β- decay B. β+ decay C. α decay D. electron capture 29. Consider the organic molecule on the right. What name most specifically describes this biochemical? A. unsaturated triglyceride B. lipid C. saturated triglyceride D. cholesterol 30. Match the functional group with its structure: x. ketone 1. y. aldehyde 2. z. ester 3. A. B. C. D. x-1, y-3, z-2 x-2, y-1, z-3 x-3, y-1, z-2 x-3, y-2, z-1 2014-15-– Science –High School – Practice Questions - Page 5 What type of decay best 31. Johannes Kepler is credited with describing planetary motion. Which of his laws can be described by the statement: a planet orbits the Sun in an elliptical orbit, with the Sun being located at a focus? A. Kepler’s First Law of Planetary Motion B. Kepler’s Second Law of Planetary Motion C. Kepler’s Third Law of Planetary Motion D. Kepler’s Fourth Law of Planetary Motion 32. Consider the diagram to the right showing projectile motion. At what angle must a projectile be launched to travel the same distance as the same object launched at 60° with the same force? A. 15° B. 22° C. 30° D. 45° 33. Often referred to as “Big G,” the universal gravitation constant was described by Isaac Newton to use in determining the gravitational force between two masses. In SI units, what unit describes Big G? A. N·m/kg2 B. N·kg/m2 C. N· (m/kg)2 D. kg·m/s2 34. Which law of thermodynamics best describes that entropy of a system will be zero at Absolute Zero? A. Zeroth law B. First law C. Second law D. Third law 35. Choose the best estimate. A crate is pulled by a rope at an angle of 45° (see diagram to the right). If 20 N of force is used to move the crate, how much work is done after the crate moves 5 m? A. 45 J B. 50 J C. 70 J D. 100 J 36. The visible spectrum is a form of electromagnetic radiation. What value is the closest approximation to the wavelength of violet light? A. 400 nm B. 7000 nm C. 500,000 nm D. 20,000,000 nm 37. Consider the diagram of a waveform on the right. What characteristic of a wave is represented by “X?” A. frequency B. trough C. amplitude D. wavelength 2014-15-– Science –High School – Practice Questions - Page 6 38. Discovered by Michael Faraday in 1831, this phenomenon produces an electromotive force when a conductor is exposed to a moving magnetic field. What is this phenomenon, which can be measured in Wb/A? A. capacitance B. inductance C. impedance D. conductance 39. An electron is a negatively charged subatomic particle. What is the charge of an electron? A. -1.6 X 10-27 C B. -4.2 X 102 C C. -1.6 X 10-19 C D. -9.1 X 10-31 C 40. Match the elementary particle with its classification: x. tau neutrino y. strange particle z. gluon A. B. C. D. 1. quark 2. lepton 3. gauge boson x-1, y-3, z-2 x-2, y-1, z-3 x-3, y-1, z-2 x-3, y-2, z-1 41. Gravitational lensing is an astronomical phenomenon in which a massive object bends light around it as the light travels towards an observer. What form of gravitational lensing is observed in the Hubble Space Telescope photo on the right? A. St. Elmo’s fire B. Doppler effect C. Galilean transformation D. Einstein’s ring 42. Coined by Max Planck, this quantity is often described as a “packet of energy.” What quantity is exemplified by a single photon of light? A. scintilla B. slug C. prius D. quantum 43. These entities can reach apparent magnitudes of -14 and brighter. What term is used for an extremely bright meteor as seen from Earth? A. tektite B. bolide C. Perseid D. baetylus 44. The Mercury Seven was a group of seven Mercury Program astronauts, who were the first American astronauts in the U.S. space program. Who of the following was NOT among the Mercury Seven? A. Alan Shepard B. John Glenn C. Deke Slayton D. Neil Armstrong 2014-15-– Science –High School – Practice Questions - Page 7 45. Surprisingly, the color of these entities may vary greatly. What celestial object has mass that is too low to sustain hydrogen fusion and typically has mass heavier than 13 Jupiter masses? A. white dwarf B. red dwarf C. blue dwarf D. brown dwarf 46. Although not tornadoes, these weather phenomena may cause as much if not more damage. What extreme downdraft of air occurs during some violent thunderstorms? A. gustnado B. microburst C. squall D. mesocyclone 47. This phenomenon occurs as a periodic warming of the waters along the western coast of South America. What is this phenomenon that can alter weather patterns globally? A. La Nina B. monsoon C. Koppen zone D. ENSO 48. Metamorphic rocks are formed under extreme pressure and heat within Earth’s crust. Which of the following is a type of metamorphic rock? A. shale B. gneiss C. breccia D. basalt 49. This Russian physiologist was studying the digestive systems of mammals when he designed the famous experiment with a canine. Who explained classical conditioning by ringing a bell to initiate salivation in a dog? A. Ivan Pavlov B. Konrad Lorenz C. Niko Tinbergen D. E. O. Wilson 50. Match the scientist with the element he discovered: x. Henry Cavendish y. Humphry Davy z. Jons Jacob Berzelius A. B. C. D. 1. Hydrogen 2. Silicon 3. Potassium x-1, y-3, z-2 x-2, y-1, z-3 x-2, y-3, z-1 x-3, y-2, z-1 2014-15-– Science –High School – Practice Questions - Page 8 Science – High School – Practice Questions 2014-15-14 1. A 26. A 2. C 27. D 3. B 28. B 4. D 29. A 5. A 30. C 6. A 31. A 7. B 32. C 8. C 33. C 9. A 34. D 10. D 35. C 11. B 36. A 12. B 37. C 13. C 38. B 14. A 39. C 15. D 40. B 16. C 41. D 17. D 42. D 18. C 43. B 19. B 44. D 20. A 45. D 21. D 46. B 22. B 47. D 23. A 48. B 24. D 49. A 25. C 50. A