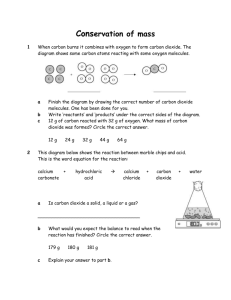
factors affecting solubility[1]
advertisement
![factors affecting solubility[1]](http://s3.studylib.net/store/data/006845945_1-dba6ad7d5483c4a4cd16b3eb01badf19-768x994.png)
Beijing World Youth Academy Candidate number: 000791 011 YeiYoung Choo Experiment No ____ 2.0 hours D1=2,D2=1,D3=0 The Effect of Temperature on the Solubility of Carbon Dioxide in Water under Constant Pressure Beijing World Youth Academy Subject: Chemistry Student name: YeiYoung Choo Candidate number: 000791 011 January 21, 2009 Teacher Helen Xu Beijing World Youth Academy Candidate number: 000791 011 YeiYoung Choo The Effect of Temperature on the Solubility of Carbon Dioxide in Water under Constant Pressure (Evaluated for Design) Research Question What is the effect of temperature on the solubility of carbon dioxide gas in de-ionised water under constant pressure? Introduction Temperature is known as one of the factors that affect the solubility of a gas in its solvent. Because the enthalpy of solution for gases dissolved in waters is usually negative (Reger, 2009; 482), students may hypothesize that the increase in the temperature will decrease the solubility of carbon dioxide in water and the decrease in the temperature will increase the solubility of carbon dioxide in water. In this experiment, the solubility of carbon dioxide in the water of selected temperatures—0°C, 20°C, 40°C and 60°C—will be tested by using the carbon dioxide gas prepared from the reaction between calcium carbonate and hydrochloric acid and dissolving it in deionised water within a gas syringe. In regards to the temperature, the gas will not be heated directly but the temperature will be manipulated according to the temperature of the solvent, which is de-ionised water. The temperature gas will be affected when thoroughly shaking the deionised water of a selected temperature with it in the gas syringe and also when putting the gas syringe into the beaker in which there would be heat transfer through the wall of the barrel (which is made of glass). As for pressure, even though the pressure will not be measured to keep constant in the experiment, it will be kept controlled for each trial of dissolving carbon dioxide by filling the same amount of gas in a gas syringe and filling in the same amount of de-ionised water in addition. Beijing World Youth Academy Candidate number: 000791 011 YeiYoung Choo Variables Independent variable Temperature under which carbon dioxide Manipulating the temperature of the dewill be dissolved in water ionised water by heating or cooling to produce de-ionised waters of selected temperatures: 0°C, 20°C, 40°C, 60°C Dependent variable Solubility of carbon dioxide in water Dissolve carbon dioxide in water within a gas syringe by shaking and measure the volume of undissolved carbon dioxide by pressing the plunger Controlled variables How to control Characteristic/Nature of the solvent (water) Using de-ionised water for every trial of dissolving carbon dioxide in it Temperature under which carbon dioxide All gas syringes filled with carbon gas is kept before dissolving in water dioxide left under room temperature Pressure under which carbon dioxide is 30mL of carbon dioxide gas thoroughly dissolved in water shaken with 20mL of water within a 60mL gas syringe for every trial Volume of water used to dissolve carbon 20mL for every trial dioxide in the gas syringe Condition of preparing carbon dioxide Carbon dioxide gas produced by reacting calcium carbonate with hydrochloric acid under room temperature Beijing World Youth Academy Candidate number: 000791 011 YeiYoung Choo Materials required Chemical 1M Hydrochloric acid (De-ionised water)1 Calcium carbonate / Marble chips Material Digital scale Conical flask Graduated beaker Bung (with a hole through which a glass tube is inserted) Rubber stopper Rubber tube (Long; approximately 30cm) Rubber tube (Short: approximately 5cm) Funnel Clamp&Stand Pinch clamp Alcohol lamp Gauze heat-proof mesh Tripod stand Glass rod Beaker tong Container Ice Glass gas syringe Thermometer Safety material Safety glasses2 Neoprene gloves3 Quantity 1 1 5 1 Uncertainty ±0.01g 25ml; 150ml; ±25ml 1 2 1 1 1 1 1 1 1 1 1 1 13 1 60mL±1mL ±1°C Quantity 1 1 Method Part (a) Preparing Gas Syringes Filled with Carbon Dioxide 1 Determining the solubility of gas requires purification of the liquid solvent and thorough degassing of the solvent. Hefter, G.. T. and Reginald P. T. Tomkins, The Experimental Determination of Solubilities. ed 3 (New York: John Wiley and Sons, 2003), 102. 2 Must wear safety glasses because hydrochloric acid is toxic and corrosive. Oxford University. “Chemical Safety Data: Hydrochloric Acid.” Dr Hugh Cartwright - Hands-on Science. http://cartwright.chem.ox.ac.uk/hsci/chemicals/hydrochloric_acid.html (accessed January 30, 2010). 3 Helpful to wear safety gloves even though the concentration is not too high. Oxford University, “Chemical Safety Data: Hydrochloric Acid.” Dr Hugh Cartwright - Hands-on Science, http://cartwright.chem.ox.ac.uk/hsci/chemicals/hydrochloric_acid.html (accessed January 30, 2010). Beijing World Youth Academy Candidate number: 000791 011 YeiYoung Choo 1. Weigh 10g of marble chips (calcium carbonate) on a digital scale. 2. Put the marble chips into the conical flask 3. Seal the flask with a bung (with a glass tube inserted in the middle) and set up the apparatus for gas collection as shown below: Figure 1. Diagrammic representation of how the apparatus should be set up to collect carbon dioxide gas, produced from a reaction between hydrochloric acid and calcium carbonate, in a gas syringe funnel rubber tube 1M hydrochloric acid pinch clamp glass tube stoppered conical flask calcium carbonate gas syringe* *Make sure the plunger of the gas syringe is pushed inward to maximum. 4. Open the pinch clamp and let the 1M hydrochloric acid flow down into the conical flask containing marble chips. 5. Leave the pinch clamp open until almost all hydrochloric acid flows down. 6. Close the clamp when there is very small amount of hydrochloric acid left in the funnel but enough to prevent any gas entering. 7. Pour more hydrochloric acid to the funnel. 8. Repeat steps 4. to 7. to continue collecting the gas produced by the reaction between calcium carbonate and hydrochloric acid, until 45mL of gas is filled in the gas syringe, as indicated by to what number on the gas syringe the plunger has been pushed outward. 9. Pull out the gas syringe from the rubber tube and, with the syringe still headed upward, push the plunger up until the volume of gas in it reaches 30mL, and quickly seal with a rubber stopper. 10. Get another gas syringe with its plunger pushed inward to maximum. 11. Hold the conical flask and move slightly to mingle the marble chips, so as to make sure more of the unreacted marble chips are exposed for the next reaction with Beijing World Youth Academy Candidate number: 000791 011 YeiYoung Choo hydrochloric acid. 12. Place the conical flask back to its position as shown previously in figure 1. 13. Repeat steps 4. to 9. 14. Repeat steps 10. to 13. to fill one more gas syringe so that three gas syringes containing the same gas are prepared. 15. Repeat steps 1. to 14. to produce ten more gas syringes filled with gas. Part (b) Dissolving carbon dioxide in de-ionised water of 20°C 16. Fill 100mL of de-ionised water in a graduated beaker. 17. Check the temperature of the water with a thermometer inserted in it and if it is higher than 20°C, holding the beaker tilted, run cold tap water along the surface of the beaker to reach 20±1°C on the thermometer. 18. Get one of the gas syringes prepared in Part (a) and hold it inversely, very close to the de-ionised water in the beaker. 19. Remove the rubber stopper and immediately carry out the next step, as shown below in figure 2. 20. Put the mouth of the gas syringe into the water and pull the plunger to fill the 20mL of water so that the end of the plunger reads 60mL on the graduation of the gas syringe. Figure 2. Diagrammic representation of how the gas syringe should be placed in the beaker containing de-ionised water to fill it with 20mL of water in addition to the 30mL of gas already filled in. pull gas syringe carbon dioxide gas de-ionised water being filled in de-ionised water 21. Pull the gas syringe out of the beaker, stopper it again and shake thoroughly to make the gas dissolve in water. 22. Pushing the stopper to make sure it is firmly attached to the mouth of the syringe, press the plunger inward. Beijing World Youth Academy Candidate number: 000791 011 YeiYoung Choo 23. Push until the plunger does not go further inward. 24. See where the end of the plunger has reached in the gas cylinder. 25. Repeat steps 17. to 24. two more times to produce three data for the de-ionised water of (about) 20°C. Part (c) Dissolving carbon dioxide in de-ionised water of 40°C 26. Fill in a graduated beaker with 100mL of de-ionised water. 27. Heat the water to 40°C with an alcohol lamp by setting up the apparatus as shown in figure 3 below: Figure 3. Diagrammic representation of how the apparatus should be set up to heat the de-ionized water, in which carbon dioxide gas would be dissolved afterwards in a gas syringe, to a selected temperature with the aid of temperature measurement by thermometer thermometer graduated beaker De-ionised water gauze heat-proof mesh tripod stand alcohol lamp 28. Stir the de-ionised water with a glass rod often during the heating process. 29. When the thermometer reads 40°C, carry the beaker out of the heating apparatus with a beaker tong. 30. Same as carried out in Part (b), repeat steps 18. to 24. to dissolve the gas in the syringe in the heated de-ionised water. 31. Check the thermometer and if the temperature has gone down, place the beaker back on the tripod stand using a beaker tong and heat up to 40°C. 32. Repeat steps 29. to 30. to produce another reading for dissolving carbon dioxide in the heated water. 33. Check the thermometer again as done in step 31. and repeat steps 29. to 30. to produce the third reading. Part (d) Dissolving carbon dioxide in de-ionised water of 60°C Beijing World Youth Academy Candidate number: 000791 011 YeiYoung Choo 34. Fill in a graduated beaker with 100mL of de-ionised water. 35. Heat the water to 60°C with an alcohol lamp by setting up the apparatus as shown in figure 3 in Part (c). 36. When the thermometer reads 60°C, carry the beaker out of the heating apparatus with a beaker tong. 37. Same as carried out in Part (b), repeat steps 18. to 24. to dissolve the gas in the syringe in the heated de-ionised water. 38. Check the thermometer and if the temperature has gone down, place the beaker back on the tripod stand using a beaker tong and heat up to 60°C. 39. Repeat steps 36. to 37. to produce another reading for dissolving carbon dioxide in the heated water. 40. Check the thermometer again as done in step 38, and repeat steps 36. to 37. to produce the third reading. Part (e) Dissolving carbon dioxide in de-ionised water of 0°C 41. Fill in a graduated beaker with 100mL of de-ionised water. 42. Fill in about 3/4 of a container with tap water and put ice as shown below in figure 4. 43. Place a thermometer in the beaker from step 41. and place the beaker in the container to cool down the de-ionised water, as shown below in figure 4. Figure 4. Diagrammic representation of preparing de-ionised water of 0°C thermometer graduated beaker de-ionised water ice container 44. Check the thermometer and wait until the temperature goes down to 0°C. 45. If the temperature stops decreasing, put some more ice in the container. 46. During the process of cooling down the de-ionised water, stir it with a stirring rod often. 47. When the thermometer reads 0°C, carry the beaker out of the container using a beaker tong. 48. Same as carried out in Part (b), repeat steps 18. to 24. to dissolve the gas in the syringe in the de-ionised water. 49. Check the thermometer and if the temperature has increased, place the beaker back Beijing World Youth Academy Candidate number: 000791 011 YeiYoung Choo into the container. 50. Take out the graduated beaker the thermometer reads 0°C. 51. Repeat steps 48. to produce another reading for dissolving carbon dioxide in the deionised water. 52. Repeat step 49. to 51. to produce the third reading. Part (f) Measuring the mass of carbon dioxide 53. Notice that there is one gas syringe from part (a) left unused. 54. Measure the mass of the gas syringe with its stopper on a digital balance as shown below in figure 5. Figure 5. Digrammic representation of measuring the mass [g] the gas syringe filled with carbon dioxide gas produced by the reaction between calcium carbonate and hydrochloric acid gas syringe carbon dioxide gas stopper digital balance 55. Remove the stopper and push the plunger the inward to get rid of carbon dioxide gas within the gas syringe. 56. Weigh the empty gas syringe and the stopper together on the digital balance. Bibliography Hefter, G.. T. and Reginald P. T. Tomkins, The Experimental Determination of Solubilities. ed 3. New York: John Wiley and Sons, 2003. Beijing World Youth Academy Candidate number: 000791 011 YeiYoung Choo Oxford University. “Chemical Safety Data: Hydrochloric Acid.” Dr Hugh Cartwright Hands-on Science. http://cartwright.chem.ox.ac.uk/hsci/chemicals/hydrochloric_acid.html (accessed January 30, 2010). Reger, Daneil L., Scott R. Goode, and David W. Ball. Chemistry: Principles and Practice. New York: Cengage Learning, 2009.