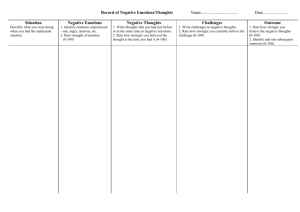
Complications of protocols that induce specific moods or emotions
advertisement

Inducing Moods or Emotions from Psychlopedia. Overview Researchers often want to explore the effects of moods or emotions on various outcoms. They might, for example, want to explore the impact of mood on creativity (e.g., De Dreu, Baas, Nijstad, 2008). To achieve this goal, researchers need to manipulate the mood or emotions of their participants. Explicit methods Often, to fulfill this goal, researchers present emotional stimuli, such as uplifting music, upsetting images, or critical feedback. Alternatively, participants are instructed to form images of emotional memories or hypothetical events (Ruys & Stapel, 2008). In both instances, the instructions or stimuli are observable. Forming images Many researchers, for example, instruct participants to generate emotional mental images (e.g., De Dreu, Baas, Nijstad, 2008; DeSteno, Petty, Rucker, Wegener, & Braverman, 2004; Strack, Schwarz, & Gschneidinger, 1985). For example, in the study conducted by De Dreu et al. (2008), participants were instructed to write a short essay about an event they experienced that provoked specific feelings--such as anger, sadness, happiness, or relaxation depending the condition to which they were assigned. They received an empty page to write this essay. After completing this task, participants were instructed to report keywords or phrases that evoked the designated feelings. To assess the validity of this manipulation, the researchers examined whether or not the essays seemed to align with the designated feelings. In addition, the length of these essays, as well as the topic, did not seem to vary across conditions. Finally, the feelings that participants wrote about did affect creativity on a subsequent task, as predicted. Neurophysiological evidence of forming images Colibazzi, Posner, Wang, Gorman, Gerber, Yu, and et al. (2010) utilized a similar procedure to De Dreu, Baas, Nijstad (2008). Participants received a sentence that was intended to evoke a specific emotion. An example might be "Imagine that you just won the lottery and you will have all the money you could ever want". They were encouraged to experience the affective state as if they were actually embedded within the context, without closing their eyes. The extent to which the sentences revolved around pleasant and unpleasant events was varied. Similarly, the extent to which the sentences revolved around activating or deactivating events was also manipulated. As evidence of validity, an fMRI study, using BOLD signals, showed that valence and arousal levels of emotions activated distinct regions. Unpleasant emotions, like sadness and stress, increased activation of the supplementary motor cortex and mid-cingulate cortex. Both of these regions connect affective representations in the limbic system to motor plans. For example, the supplementary motor cortex translates affective cues to sequences of movement--in contrast to the lateral prefrontal cortex that translates external cues to sequences of movement (Kandel, Schwartz, & Jessell, 2000). Similarly, the anterior portion of the mid-cingulate cortex translates anticipated negative consequences to motor plans (Vogt, Berger, & Derbyshire, 2003). Furthermore, these unpleasant emotions also activated the dorsolateral prefrontal cortex and the inferior parietal cortex. These two regions are regarded as key facets of the dorsal attentional system, essential to the role of maintaining attention on stimuli that coincide with goals, particularly in threatening contexts (Corbetta & Shulman, 2002). In addition, the dorsolateral prefrontal cortex underpins the suppression and regulation of emotions (e.g., Levesque, Joanette, Mensour, Beaudoin, Leroux, Bourgouin, & Beauregard, 2004). In short, this region might be activated to direct attention to goals, partly to regulate unpleasant emotions. Unpleasant emotions also increased activation of the right temporo-occipital junction. This region represents part of the ventral visual stream. This stream connects visual areas to the medial temporal lobe, to store visual memories, especially negative events (see Godinho, Magnin, Frot, Perchet, & Garcia-Larrea, 2006). Finally, unpleasant emotions increased activation of the right dorsal cerebellum, critical to regulate motor plans. In short, negative emotions seem to mobilize circuits that connect emotion to motor programs as well as maintain attention to broader goals (Colibazzi, Posner, Wang, Gorman, Gerber, Yu, & et al., 2010). After a transient delay, pleasant emotions activated regions in the mesolimbic dopaminergic system, underpinning reward. These regions include the substantia nigra, in the midbrain, as well as the ventral striatum and right caudate (Colibazzi, Posner, Wang, Gorman, Gerber, Yu, & et al., 2010). In contrast to valence, the level of arousal or activation of emotions was primarily associated with activation in the medial temporal lobe, such as the amygdala and hippocampus, together with the basal ganglia and thalamic nuclei (Colibazzi, Posner, Wang, Gorman, Gerber, Yu, & et al., 2010). Specifically, activated emotions, like tension or excitement, were associated with activation of the amygdala. These emotions also activated the hippocampus and para- hippocampus, both involved in memory retention. This activation of the amygdala, hippocampus, and para-hippocampus aligns with the argument that emotional arousal facilitates the consolidation of emotional memories (e.g., LaBar & Cabeza, 2006). The thalamus, together with other midline structures mediates, information from the hippocampus to the medial prefrontal cortex, arguably to alert individuals to stimuli that are relevant (Vertes, Hoover, Szigeti-Buck, & Leranth, 2007). Consistent with this argument, arousing emotions activated the thalamus in this study (Colibazzi, Posner, Wang, Gorman, Gerber, Yu, & et al., 2010). Furthermore, arousing emotions activated the left caudate and the globus pallidus. These two part of the basal ganglia associate stimuli and responses, primarily to facilitate the formation of automatic behavior. Thus, these regions might encode procedures that are especially germane to emotional contexts (Colibazzi, Posner, Wang, Gorman, Gerber, Yu, & et al., 2010). Finally, whereas unpleasant stimuli were more likely to activate the right hemisphere, arousing stimuli were more likely to activate the left hemisphere. Broadly, these findings vindicate the circumplex model of emotions (e.g., Posner, Russell, & Peterson, 2005). According to this model, most emotions can be represented as a function of two dimensions: valence and activation. For example, unpleasant emotions with limited activation include sadness, depression, boredom and fatigue. Unpleasant emotions with elevated activation include tension, stress, and a feeling of being upset. Pleasant emotions with limited activation include calmness, relaxation, serenity, and contentment. Pleasant emotions with considerable activation include alertness, excitement, elation, and happiness. These emotions can be arranged in a circle. This model departs from the theory of basic or discrete emotions, in which each emotion is assumed to represent a specific neural system (Ekman, 1992). Movies Many researchers, to manipulate mood, also present movie clips to participants. For example, Forgas and East (2008) presented a 10 minute extract from a comedy series to promote happiness, a nature documentary to promote a neutral mood, and a film about cancer to promote a sad mood--a technique that was validated by Forgas (2002). To validate this manipulation, after completing the other procedures in the study, participants were asked to specify how they felt on an 8 point scale, from good to bad or from happy to sad. Mood, as gauged by these two items, was highest after watching the happy clip and lowest after watching the sad clip. In many studies, participants are instructed to read stories that are either uplifting, distressing, or neutral. For example, Erber (1991) as well as Avramova and Stapel (2008) presented one of three stories in each participants. To induce a positive mood, one of the stories was about a young artist who ultimately receives a scholarship to study art. To induce a negative mood, the same artist was impaired by a disabling illness, such as arthritis. Finally, to induce a neutral mood, the story described a young artist who decides which college to attend. In a pilot study, conducted by Avramova and Stapel (2008), participants specified the extent to which they feel positive or negative on a nine point semantic differential scale. This study showed these stories do affect mood as intended, with an eta squared value of.32. Music Music is often used to influence the mood of participants. In the study conducted by Pedersen, Bushman, Vasquez, and Miller (2008), for example, some participants heard loud distracting music, intended to provoke anger. In particular, they listened to Firebird Suite, by Stravinski, at 80 dB. Other participants heard soothing music to promote relaxation. They listened to Interludes by Mannheim Steamrller played at 70 dB. Likewise, to induce a positive mood, Avramova and Stapel (2008) presented allegros from Mozart's Eine kleine Nachtmusik over headphones. To induce a negative mood, participants heard Barber's Adagio for Strings instead. Subsequently, participants specified the extent to which they feel positive or negative on a seven point semantic differential scale. Music did affect mood as intended, with an eta squared value of .30. Feedback Various forms of feedback can be presented to evoke specific affective states. For example, in a study conducted by Pedersen, Bushman, Vasquez, and Miller (2008), participants completed a series of anagrams and verbalized their answers through an intercom system. To provoke negative affective states, the experimenter sometimes acted insultingly. After the fourth answer, for example, the experimenter said "Look I can barely hear you." The feedback was even more provocative after the eighth and twelfth answer, culiminating in the remark "Can't you follow directions? Speak louder". Implicit methods Alternatively, some researchers have developed techniques in which the stimuli are not observable or intuitively related to mood or emotions. Masked primes In a study conducted by Ruys and Stapel (2008), for example, participants were exposed to a set of subliminal words. In particular, words were presented on a screen, but towards the periphery. These words either represented positive terms, such as confident, persistent, honest, and pleasant, or negative terms, such as arrogant, dishonest, and wrong. The words appeared on the screen for 40 ms or 120 ms, and were then followed by a mask that lasted 120 ms (for details, see Stapel, Koomen, & Ruys, 2002). Participants only recognized a flash and could not read the words. Their task was to identify whether the words appeared on the left or right of the screen. Words that lasted only 40 ms were especially likely to affect mood (Ruys & Stapel, 2008). Specifically, these words were especially likely to affect whether or not participants claimed they felt positive or negative emotions and also affected their processing style. That is, negative words that lasted only 40 ms encouraged a more deliberate, analytical, and exhaustive processing style, consistent with dual process theories. To explain this finding, Ruys and Stapel (2008) argued that words evoke two sets of cognitive processes. First, individuals evaluate whether a word, such as "honest", is positive or negative. A positive evaluation improves mood and a negative evaluation compromises mood. Furthermore, individuals identify unemotional information about this word. They might, for example, recognize that "honest" is a short term, is not associated with politicians, is related to truth, is the opposite to dishonest, and so forth. This information tends to curb emotions. Usually, evaluations are formed more rapidly than descriptive information is identified, as affective primacy theory implies (Zajonc, 1980, 2000). Thus, words that last 40 ms appear long enough to promote evaluation, which influences emotions, but not long enough to promote description, which tends to diminish emotions. Complications of protocols that induce specific moods or emotions Many of the protocols that are used to induce moods, such as observing photographs, watching movies, or remembering past events, can be challenged. That is, these protocols also determine which cognitive operations or contexts are evoked rather than merely elicit specific emotions. Hence, these operations or contexts, instead of the emotions themselves, might explain the effects of these protocols (for a discussion, see Chemali, Chahine, & Naasan, 2008). References Avramova, Y. R., & Stapel, D. A. (2008). Mood as spotlights: The influence of mood on accessibility effects. Journal of Personality and Individual Differences, 95, 542-554. Chemali, Z. N., Chahine, L. M., & Naasan, G. (2008). On happiness: A minimalist perspective on a complex neural circuitry and its psychosocial constructs. Journal of Happiness Studies, 9, 489-501. Colibazzi, T., Posner, J., Wang, Z., Gorman, D., Gerber, A., Yu, S., & et al. (2010). Neural systems subserving valence and arousal during the experience of induced emotions. Emotion, 10, 377-389. Corbetta, M., & Shulman, G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3, 201-215. Damasio, A., Grabowski, T., Bechara, A., Damasio, H., Ponto, L., Parvizi, J., & Hichwa, R. (2000). Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience, 3, 1049-1056. De Dreu, C. K. W., Baas, M., Nijstad, B. A. (2008). Hedonic tone and activation level in the moodcreativity link: toward a dual pathway to creativity model. Journal of Personality and Social Psychology, 94, 739-756. DeSteno, D., Petty, R. E., Rucker, D. D., Wegener, D. T., & Braverman, J. (2004). Discrete emotions and persuasion: The role of emotion-induced expectancies.Journal of Personality and Social Psychology, 86, 43-56. Ekman, P. (1992b). Are there basic emotions? Psychological Review, 99, 550-553. Erber, R. (1991). Affective and semantic priming: Effects of mood on categoriy accessibility and inference. Journal of Experimental Social Psychology, 27, 480-498. Forgas, J. P. (2002). Feeling and doing: Affective influences on interpersonal behavior, Psychological Inquiry, 13, 1-28. Forgas, J. P., & East, R. (2008). On being happy and gullible: Mood effects on scepticism and the detection of deception. Journal of Experimental Social Psychology, 44, 1362-1367. George, M. S., Ketter, T. A., Parekh, P. I., Horwitz, B., Herscovitch, P., & Post, R. (1995). Brain activity during transient sadness and happiness in health women. The American Journal of Psychiatry, 152, 341351. Godinho, F., Magnin, M., Frot, M., Perchet, C., & Garcia-Larrea, L. (2006). Emotional modulation of pain: Is it the sensation or what we recall? Journal of Neuroscience, 26, 11454-11461. Kandel, E. R., Schwartz, J. H., & Jessell, T. M. (2000). Principles of neural science (4th ed.). New York: McGraw-Hill, Health Professions Division. LaBar, K. S., & Cabeza, R. (2006). Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience, 7, 54-64. Lane, R., Reiman, E., Ahern, G., Schwartz, G., & Davidson, R. (1997). Neuroanatomical correlates of happiness, sadness and disgust. The American Journal of Psychiatry, 154, 926-933. Levesque, J., Joanette, Y., Mensour, B., Beaudoin, G., Leroux, J. M., Bourgouin, P., & Beauregard, M. (2004). Neural basis of emotional self-regulation in childhood. Neuroscience, 129, 361-369. Pedersen, W. C., Bushman, B. J., Vasquez, E. A., & Miller, N. (2008). Kicking the (barking) dog effect: The moderating role of target attributes on triggered displaced aggression. Personality and Social Psychology Bulletin, 34, 1382-1395. Posner, J., Russell, J. A., & Peterson, B. S. (2005). The circumplex model of affect: An integrative approach to affective neuroscience, cognitive development, and psychopathology. Development and Psychopathology, 17, 715-734. Ruys, K. I., & Stapel, D. A. (2008). How to heat up from the cold: examining the preconditions for (unconscious) mood effects. Journal of Personality and Social Psychology, 94, 777-791. Stapel, D. A., Koomen, W., & Ruys, K. I. (2002). The effects of diffuse and distinct affect. Journal of Personality and Social Psychology, 83, 60-74. Strack, F., Schwarz, N., & Gschneidinger, E. (1985). Happiness and reminiscing: The role of time perspective, affect, and mode of thinking. Journal of Personalityand Social Psychology, 49, 1460-1469. Vertes, R. P., Hoover, W. B., Szigeti-Buck, K., & Leranth, C. (2007). Nucleus reuniens of the midline thalamus: Link between the medial prefrontal cortex and the hippocampus. Brain Research Bulletin, 71, 601-609. Vogt, B. A., Berger, G. R., & Derbyshire, S. W. (2003). Structural and functional dichotomy of human midcingulate cortex. European Journal of Neuroscience, 18, 3134-3144. Zajonc, R. B. (1980). Feeling and thinking: Preferences need no inferences. American Psychologist, 35, 151-175. Zajonc, R. B. (2000). Feeling and thinking: Closing the debate over the independence of affect. In J. Forgas (Ed.), Feeling and thinking: The role of affect in social cognition (pp. 31-58). New York: Cambridge University Press.