Biosensors Based on Graphene Modified Screen
advertisement

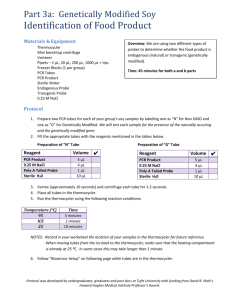
Biosensors Based on Graphene Modified Screen-Printed Electrodes for The Detection of Catecholamines Received for publication, March 25, 2014 Accepted, June 14, 2014 I. M. APETREIa, C. V. POPA (UNGUREANU) b, C. APETREIc,*, D. TUTUNARUa a Department of Pharmaceutical Sciences, Faculty of Medicine and Pharmacy, “Dunarea de Jos” University of Galati b Faculty of Food Science and Engineering, “Dunarea de Jos” University of Galati c Department of Chemistry, Physics and Environment, Faculty of Sciences and Environment, “Dunarea de Jos” University of Galati *Address correspondence to: “Dunarea de Jos” University of Galati, Faculty of Sciences and Environment, 47 Domneasca Street, 800201, Galati, Romania; E-mail: apetreic@ugal.ro Abstract This work reports tyrosinase based electrochemical biosensors using graphene modified screen-printed carbon based electrodes for the determination of catecholamines. The enzyme has been immobilized onto the graphene modified carbon working electrodes by cross-linking with glutaraldehyde. The detection has been performed by measuring the cathodic current due to the reduction of the corresponding quinone a low potential, 0.025 V for dopamine and -0.025 V for epinephrine, respectively. The experimental conditions for the tyrosinase immobilization and the main variables that can influence the cathodic current have been optimized. Under optimum conditions, the electrochemical biosensors have been characterized. A linear response range from 0.2 up to 25 M of dopamine and from 1 to 27.5 M of epinephrine was obtained. The detection limits are in the range 2.42×10-7 - 6.56×10-7 M for developed biosensors. The biosensors construction was highly reproducible. Finally, the developed biosensors have been applied to the determination of dopamine and epinephrine content in pharmaceutical formulation samples. Keywords: biosensor, screen-printed electrode, tyrosinase, graphene, catecholamine 1. Introduction Catecholamines (dopamine, epinephrine and norepinephrine) play a major role in the nervous system as central and peripheral neurotransmitters [1]. Catecholamines are biological amines released mainly from the adrenal glands in response to stress, acting as neurotransmitters. Therefore, they are maintaining normal physical activity of the body including blood pressure, heart rate and the reactions of the sympathetic nervous system [2]. The identification and quantification of catecholamines in biological fluids is of great importance in medical diagnostics, principally for patients suffering from Parkinson's disease and stress. Consequently, there is a necessity for their quantitative determination in pharmaceutical products and biological fluids for detecting any endocrine disorders or any other physiological and pathological abnormality in the body [3]. Various techniques have been implemented for the determination of catecholamines such as high performance liquid chromatography [4], capillary electrophoresis [5], chemiluminescence [6] and fluorescence [7]. Usually, these methods are relative complicated as they need complex derivatization procedures or combination of various detection methods. Furthermore, some of these methods do not have enough sensitivity and selectivity in the 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 corresponding determinations. Contrary, electrochemical detection methods have shown several advantages such as high sensitivity, low cost, not time consuming and simple surface modification of electrodes. As a consequence of these advantages, several electrochemical methods are available for the determination of dopamine (DA) [8-10] and epinephrine (EP) [11-13]. Biosensors, which combine biological recognition through enzyme specificity with construction simplicity, have been reported as a good and cheap alternative for the traditional ones [14]. The use of disposable transducers, such as screen-printed electrodes (SPEs), boosts the intrinsic characteristics of biosensors. Screen-printing technology, which offers design flexibility, process automatization and good reproducibility in the transducers fabrication, had been shown as a method for mass production of biosensors at low cost [15]. In the case of biogenic amines, however, some results have been described in the literature. Lange and Wittmann [16], as well as Chemnitius and Bilitewski [17] have described amino oxidase-sensors based on platinum screen printed electrodes, using a glutaraldehyde - bovine serum albumin cross-linking immobilization procedure, for the determination of histamine, putrescine and tyramine. Alonso-Lomillo et al. report monoamine oxidase/horseradish peroxidase and diamine oxidase/horseradish peroxidase based biosensors using screen-printed carbon electrodes for the determination of several biogenic amines [18]. Apetrei et al. report the development of disposable biosensors based on carbonaceous screen-printed electrodes and diamine oxidase [19]. In this work, biosensors based on graphene modified screen-printed carbon electrodes and containing tyrosinase as biocatalyst were prepared and their capability to detect dopamine and epinephrine was evaluated. For this purpose, dopamine and epinephrine detection was carried out in buffered aqueous solutions. The amperometric characteristics including kinetics, calibration curves and limits of detection in the detection of dopamine and epinephrine were investigated. The potential of biosensors to detect and quantify dopamine and epinephrine in pharmaceutical formulations was studied. 2. Materials and Methods 2.1. Chemical and solutions All reagents were of high purity and used without further purification. Dopamine and epinephrine were purchased from Sigma-Aldrich. Tyrosinase (EC 1.14.18.1, from mushroom) was purchased from Sigma. A 5 mg×mL-1 solution of tyrosinase in buffer phosphate solution (0.01 M, pH 7.0) was used for the enzyme immobilization. The phosphate buffer solutions were prepared from phosphoric acid, potassium monobasic and dibasic phosphate salts from Aldrich. Ultrapure water (18 MΩ×cm, Millipore Milli-Q) was used for preparation of all aqueous solutions. 2.2. Apparatus Electrochemical measurements were performed on a Biologic Science Instruments SP 150 potentiostat/galvanostat using the EC-Lab Express software. An UV-Vis spectrophotometer from Labomed Inc. connected to a PC (software UVWin) was used for spectrophotometric experiments. An Elmasonic S10H ultrasonic bath was used for dissolving and homogenization of solutions. For pH measurements an Inolab pH 7310 was used. For stirring of solutions in amperometric measurements a magnetic stirrer (Velp, Italy) was used. 2.3. Electrodes and electrochemical cell Screen-printed carbon electrodes (4 mm diameter, S=12.56 mm2) purchased from Dropsens, www.dropsens.com, model DRP110GPH (screen-printed carbon electrodes modified with graphene) were used for biosensor construction. A three-electrode configuration was used. The reference and the counter electrode integrated in the device were used (counter electrode - carbon, reference electrode - 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 silver). Cyclic voltammograms were registered from -0.5 to +0.5V (the scan started at 0V) at a scan rate of 0.05 V×s-1 (except otherwise indicated). 2.4. Fabrication of biosensors Ty was immobilized over graphene-carbon thick film by casting technique followed by cross-linking with glutaraldehyde. 50 L of 0.01 M phosphate buffer (pH 7.0) containing 5 mg×mL-1 of Ty was added onto 12.56 mm2 area of graphenecarbon thick film. After drying, the Ty-graphene-carbon films were exposed to a 2.5% (v/v) glutaraldehyde solution (in PBS 0.01 M of pH 7.0) for 20 min at room temperature. The enzyme-immobilized film was dried at 10°C and rinsed with phosphate buffer solution to remove any unbound enzyme from the electrode surface. Finally, the biosensors were further dried at 10°C and stored at 4°C [30]. To study the repeatability and stability of the biosensor based on tyrosinase immobilized on graphene screen-printed carbon electrode, amperometric measurements were carried out in a 10−5 M dopamine solution in 0.01 M PBS at pH 7.0, using the same biosensor device. 2.5. Pharmacopoeia method The spectrophotometric methods established in the X Romanian Pharmacopoeia (1993) [29] were used to check the accuracy of the results obtained with the proposed biosensors. 3. Results and Discussions In this study, a nanomaterial based on carbon, graphene (GPH), was used as immobilization matrix for tyrosinase (Ty). The screen-printed carbon electrodes modified with GPH are designed for the development of (bio)sensors with an enhanced electrochemical active area and enhanced electronic transfer properties. 3.1. Cyclic voltammetry Thermal The cyclic voltammograms of biosensor in 100 M dopamine solution and 100 M epinephrine (in 0.01 M phosphate buffer solution, pH 7.0), respectively, were shown in Figure 1. 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 Figure 1. Cyclic voltammogram of biosensor in a) 100 M dopamine solution (0.01 M PBS, pH 7.0). b) 100 M epinephrine solution (0.01 M PBS, pH 7.0). Scan rate 0.050 V×s-1 As can be seen, in the cyclic voltammetry of DA (a catecholamine), one oxidation peak and one reduction peak are observed (Figure 1a). The oxidation peak is due to the oxidation of catecholamine to o-quinone, which is reversible in nature: Dopamine + Tyrosinase (O2) → o-Dopaquinone + H2O The o-dopaquinone is electrochemically active is electrochemically reduced to biosensor surface: o-Dopaquinone + 2H+ + 2e- → Dopamine 125 126 127 128 129 130 131 132 EP follows the same reaction mechanism. EP gets oxidized to epinephrinequinone, which is electrochemically reduced to biosensor surface. In DA solution (supporting electrolyte PBS 0.01 M, pH 7.0), the CV gave two well defined peaks locating, one cathodic at 0.025 V, and one anodic at 0.30 V. In the presence of EP (100 M) in PBS (Figure 1b), the CV curves gave two well defined peaks locating, one cathodic at -0.025 V, and one anodic at +0.40 V. These results are in good agreement with results published using poly(indole-5-carboxylic acid)/tyrosinase electrode or carbon black/tyrosinase biosensor [20,21]. These results demonstrate that the tyrosinase enzyme retains its bioactivity to a large extent when immobilized on GPH-C thick film. Tyrosinase immobilized in GPH efficiently catalyzes the oxidation of DA or EP. The sharp and intense oxidation and reduction peaks reveal a fast electron transfer at tyrosinase immobilized GPH-C electrode [22]. Additionally, the effect of potential scan rate on the peak current of the two catecholamines was studied. From Figure 2, it can also be seen that the oxidation peak shifts to a more positive value and the reduction peak for both molecules shifts to the more negative values with increasing scan rates along with a concurrent increase in current. 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 Figure 2. (a) Cyclic voltammograms of biosensor in 100 M DA (pH 7.0) registered with different scan rates. (b) Cyclic voltammograms of biosensor in 100 M EP (pH 7.0) registered with different scan rates. The cyclic voltammetric results point out the cathodic peak currents (Ip) for both catecholamines varied linearly with the scan rate () ranging from 0.050 V×s-1 to 1.000 V×s-1 (Figure 2) which implies that the reduction of DA and EP is kinetically controlled on Ty/GPH-C/SPE. 3.2. Optimization of experimental parameters The pH of the supporting electrolyte showed a significant effect on the electrochemical behavior of DA (100 M) and EP (100 M) at the surface of the biosensor. Cyclic voltammetry (CV) was carried out to characterize the influence of pH of the buffer solution (in the range of 4.0-9.0). The influence of the pH on the oxidation peaks currents of DA and EP was investigated employing phosphate buffer solutions. It was observed that as the pH of the medium was progressively augmented, the potential kept on shifting towards less positive values. The involvement of proton in the reaction is suggested. Over the pH range of 4.0-9.0, the peak potential (Ep) for DA and EP is found to be a linear function of pH. From the plot of Ep vs. pH, slopes of 0.051, and 0.0534 V/pH unit were obtained for DA (Figure 3a) and EP (Figure 3b), respectively. These slopes reveal that an equal number of protons and electrons are involved in the reduction reactions of DA and EP. Additionally, it 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164 165 was observed that the peak current for both DA and EP was maximum at pH 7.0. Thus, these optimized pH values were employed for further studies. 166 167 Figure 3. a) A plot of peak current (ip) vs. pH and peak potential (Ep) vs. pH for 100 M DA at Ty/GPH-C/SPE employing CV; b) A plot of peak current (ip) vs. pH and peak potential (Ep) vs. pH for 100 M EP at Ty/GPH-C/SPE employing CV. The applied potential has a key influence over the biosensor response, because the applied potential contributes to the sensitivity and selectivity of the biosensor. The potential dependence on the biosensor response is shown in Figure 4 using 100 M DA in 0.01 M phosphate buffer (pH 7.0) and 100 M EP in 0.01 M phosphate buffer (pH 7.0), respectively. 168 169 170 171 172 Figure 4. Current-potential dependence in (a) 100 M DA and (b) 100 M EP (supporting electrolyte 0.01 M PBS, pH 7.0, constant stirring). The maximum of the signal is obtained at +0.025 V. In the case of EP The maximum of the signal is obtained at -0.025 V. Therefore, +0.025 V was used as the applied potential for DA detection, and -0.025 V for EP detection. 3.3. Amperometric response of biosensor Figure 5 (a) illustrates a typical amperometric response for the Ty/GPH-C/SPE biosensor at +0.025 V after the addition of successive aliquots of DA to the 0.01 M PBS (pH 7.0) under constant stirring. As presented in the Figure, a well-defined reduction current proportional to the concentration of catecholamine is observed, which results from the electrochemical reduction of o-dopaquinone species enzymatically formed. 173 174 175 176 177 178 179 180 181 182 Figure 5. (a) Amperometric response of Ty/GPH-C/SPE biosensor to DA (in 0.01 M PBS solution, pH 7.0), with levels increasing in 40 M increments; Applied potential +0.025V (b) Amperometric response of Ty/GPH-C/SPE biosensor to EP (in 0.01 M PBS solution, pH 7.0), with levels increasing in 40 M increments; Applied potential -0.025V. The Ty/GPH-C/SPE biosensor achieves 95% of steady-state current in less than 5 s. The response rate is much faster than that of 10 s reported in a Ty-conducting polymer film based biosensor [23] and comparable with that reported for a Ty-graphite paste based biosensor [24]. Such fast response is attributed to a fast electron transfer between the enzymaticallyproduced dopaquinone and the biosensor. Figure 6 (a) showed the relationship between the response current of the biosensor and the DA concentration in PBS (pH 7.0) at +0.025 V (calibration curve). 183 184 185 186 187 188 189 190 Figure 6. (a) Calibration curve between the cathodic current and the concentration of DA in 0.01 M PBS solution (pH 7.0) of Ty/GPH-C/SPE biosensor; (b) Calibration curve between the cathodic current and the concentration of EP in 0.01 M PBS solution (pH 7.0) of Ty/GPH-C/SPE biosensor. It can be seen from Figure 6 (a) that the response current is linear with DA concentration in the range from 0.2 up to 25 M with sensitivity of 0.64A/M for the cathodic process. The corresponding detection limit was calculated according to the 3sb/m criterion. In this equation m is the slope of the calibration graph. sb is the standard deviation (n = 5) of the voltammetric signals of the substrate at the concentration level corresponding to the lowest concentration of the calibration plot. The detection limit calculated was 2.42×10-7 M. The detection limit is in the range of the detection limits published for biosensors based on tyrosinase [25]. 191 192 193 194 195 196 197 From the above results, the Hill coefficient (h) can be calculated by representing the log[I/(Imax-I)] vs. log [DA] (the logarithm of the substrate concentration). A Hill coefficient of 1.04 was calculated for the enzymatic process (R2=0.9915). The finding that the h parameter, calculated from the corresponding Hill’s plot, was close to unity, demonstrated that the kinetics of the enzymatic reaction fitted into a Michaelis-Menten type kinetics [26]. The enzymatic kinetic parameters were obtained using the linearization of Lineweaver-Burk expressed by equation (1) [27]: K app 1 1 M (1) I I max I max S where [S] is the concentration of the substrate, I is the cathodic current at 0.025V, KMapp is the apparent Michaelis-Menten constant for the enzymatic reaction and Imax is the steady-state current. Using the Lineweaver-Burk equation and representing 1/I vs. 1/[DA] the apparent Michaelis-Menten constant was calculate from the slope and the Imax from the intercept. KMapp of 18.75 M and an Imax of 24.84Awere obtained for DA. KMapp is lower than the values usually reported for tyrosinase biosensors (in the range of mM) using other immobilization techniques [28]. This proves the benefits of the GPH in construction of biosensor with high quality performances. In the case of epinephrine similar results were obtained as was summarized in Table 1. The graphical results are presented in the Figures 5 (b) and 6 (b). Table 1. Response characteristics of the Ty/GPH-C/SPE biosensor to catecholamines Compound h LOD (M) Linear range (M) KMapp (M) Imax (A) -7 Dopamine 1.04 0.2 - 25 2.42×10 18.75 24.84 Epinephrine 0.96 1 - 27.5 6.56×10-7 15.56 198 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216 12.34 3.4. Repeatability and stability of the biosensor To study the repeatability and stability of the biosensor based on tyrosinase immobilized on graphene screen-printed carbon electrode, amperometric measurements were carried out in a 10−5 M dopamine solution in 0.01 M PBS at pH 7.0 using the same biosensor device. Among replicate measurements, the biosensor was tacked off from the electrolyte solution containing dopamine and rinsed with ultrapure water and 0.01 M PBS (pH 7.0). The relative standard deviation (%RSD) of 10 measurements was 4.1%. Therefore, several measurements can be carried out without a significant variation of the biosensor response, lower than 5%. The stability of the biosensor was studied by monitoring the amperometric response at regular intervals of 24 hours for 3 days. Between measurements, the biosensor was stored in a refrigerator at 4°C in 0.01 M PBS at pH 7.0. The amperometric responses of the biosensor decrease constantly. After 3 days of storage, the biosensor maintains 67% of its initial current response. Therefore, the biosensor can be used as a disposable single use device. 3.5. Application of the biosensor to determination of DA and EP in pharmaceutical formulas The UV spectrophotometric methods indicated in the X Romanian Pharmacopoeia suggest the measurement of dopamine at 280 nm, in 1 g×L-1 Na2SO3, and the measurement of epinephrine at 279 nm, in presence of 10-2 M HCl [29]. Calibration curves were constructed using pure dopamine, and epinephrine, respectively. These were used for quantification of dopamine and epinephrine, respectively, in different of pharmaceutical formulations. Table 2 shows the results obtained for the analyses of pharmaceutical formulations using the UV spectrophotometric procedure and biosensor, respectively (at +0.025 V for DA and 0.025 V for EP). 217 218 219 220 221 222 223 224 225 226 227 228 229 230 231 232 233 234 235 236 237 238 239 240 Table 2. Determination of DA and EP in pharmaceutical formulations by UV spectrophotometry and biosensor No. Compound Sample a b c 1. Dopamine Dopamine chlorhydrate (S.C. Zentiva S.A., Romania) 50 501.2 491.6 2. Dopamine Dopamin (Admeda Arzneimittel GmbH, Germany) 20 200.7 19 0.8 3. Epinephrine Adrenalina (S.C. Terapia S.A., Romania) 1 1.00.05 1.10.07 4. Epinephrine Anapen® (Lincoln Medical Limited, UK) 1 1.00.06 0.90.08 a - Amount of catecholamine in the sample (mg). b - Amount of catecholamine obtained by the proposed method (mg) RSD (n=5). c - Amount of catecholamine obtained by the spectrophotometric method (mg) RSD (n=5). RSD - Relative standard deviation 4. Conclusions A novel biosensor has been developed for the determination of catecholamines employing tyrosinase immobilized on graphene screen-printed carbon electrodes. The advantages of this biosensor comparing with traditional analytical methods are sensitivity, fabrication simplicity and short time for analysis. The proposed biosensor is very sensitive having sub-micromolar detection limits for dopamine and epinephrine. The biosensor has been employed for the determination of dopamine and epinephrine in pharmaceutical formulations and the results are satisfactory, which suggests that biosensor can act as a promising biosensor for the determination of dopamine and epinephrine. The concentration results have been in a good agreement with the Pharmacopoeia method. 241 242 243 244 245 246 247 248 249 250 251 252 253 254 255 256 257 Acknowledgments 258 This work was supported by a grant of the Romanian National Authority for Scientific Research, CNCS - UEFISCDI, project number PN-II-ID-PCE-2011-3-0255. 259 260 261 References 1. 2. 3. 4. 5. 6. 7. 8. 9. GERALD MC, in: FAROOQUI T, FAROOQUI AA (Eds.), Biogenic Amines: Pharmacological, Neurochemical, and Molecular Aspects in the CNS, Nova Biomedical Books, New York, 2010, pp. 312. KUMAR A, HART JP, MCCALLEY DV, Determination of catecholamines in urine using hydrophilic interaction chromatography with electrochemical detection, J. Chromatogr. A, 1218, 3854-3861 (2011). WHITING MJ, Simultaneous measurement of urinary metanephrines and catecholamines by liquid chromatography with tandem mass spectrometric detection, Ann. Clin. Biochem., 46, 129-136 (2009). BICKER J, FORTUNA A, ALVES G, FALCÃO A, Liquid chromatographic methods for the quantification of catecholamines and their metabolites in several biological samples-a review, Anal. Chim. Acta, 768, 12-34 (2013). LIU WL, HSU YF, LIU YW, SINGCO B, CHEN SW, HUANG HY, CHIN TY, Capillary electrophoresis-laser-induced fluorescence detection of rat brain catecholamines with microwaveassisted derivatization, Electrophoresis, 33, 3008-3011 (2012). KŁADNA A, BERCZYŃSKI P, KRUK I, MICHALSKA T, ABOUL-ENEIN HY, Superoxide anion radical scavenging property of catecholamines, Luminescence, 28, 450-455 (2013). HETTIE KS, LIU X, GILLIS KD, GLASS TE, Selective Catecholamine Recognition with NeuroSensor 521: A Fluorescent Sensor for the Visualization of Norepinephrine in Fixed and Live Cells, ACS Chem. Neurosci., 4, 918-923 (2013). QIAN T, YU C, WU S, SHEN J, In situ polymerization of highly dispersed polypyrrole on reduced graphite oxide for dopamine detection, Biosens. Bioelectron., 50, 157-160 (2013). SANGHAVI BJ, MOBIN SM, MATHUR P, LAHIRI GK, SRIVASTAVA AK, Biomimetic sensor for certain catecholamines employing copper(II) complex and silver nanoparticle modified glassy carbon paste electrode, Biosens. Bioelectron., 39, 124-132 (2013). 262 263 264 265 266 267 268 269 270 271 272 273 274 275 276 277 278 279 280 281 282 283 284 285 286 10. APETREI IM, TUTUNARU D, POPA (UNGUREANU) CV, APETREI C, Development of amperometric biosensor based on tyrosinase immobilized in phosphate-doped polypyrrole film for detection of biogenic amines, 14th International Meeting on Chemical Sensors - IMCS 2012, Nuremberg, Germany, 855-858 (2012). 11. GOYAL RN, AGRAWAL B, Ag ion irradiated based sensor for the electrochemical determination of epinephrine and 5-hydroxytryptamine in human biological fluids, Anal. Chim. Acta, 743, 33-40 (2012). 12. BRONDANI D, SCHEEREN CW, DUPONT J, VIEIRA IC, Halloysite clay nanotubes and platinum nanoparticles dispersed in ionic liquid applied in the development of a catecholamine biosensor, Analyst, 137, 3732-3739 (2012). 13. VEROLA MATAVELI LR, DE JESUS ANTUNES N, RIBEIRO PEREIRA LIMA BRIGAGÃO M, SCHMIDT DE MAGALHÃES C, WISNIEWSKI C, ORIVAL LUCCAS P, Evaluation of a simple and low cost potentiometric biosensor for pharmaceutical and in vivo adrenaline determination, Biosens. Bioelectron., 26, 798-802 (2010). 14. APETREI C., GHASEMI-VARNAMKHASTI M., in DE LA GUARDIA M., GONZÁLVEZ A. (Eds.), Food Protected Designation of Origin - Methodologies and Applications, Comprehensive Analytical Chemistry, Elsevier, vol. 60, 2013, pp. 279-297. 15. DOMÍNGUEZ-RENEDO O., ALONSO-LOMILLO M.A., ARCOS-MARTINEZ M.J., Recent developments in the field of screen-printed electrodes and their related applications, Talanta, 73, 202219 (2007). 16. LANGE J, WITTMANN C, Enzyme sensor array for the determination of biogenic amines in food samples, Anal. Bioanal. Chem., 372, 276-283 (2002). 17. CHEMNITIUS GC, BILITEWSKI U, Development of screen-printed enzyme electrodes for the estimation of fish quality, Sens. Actuators B, 32, 107-113 (1996). 18. ALONSO-LOMILLO MA, DOMÍNGUEZ-RENEDO O, MATOS P, ARCOS-MARTÍNEZ MJ, Disposable biosensors for determination of biogenic amines, Anal. Chim. Acta, 665, 26-31 (2010). 19. APETREI I, POPA (UNGUREANU) CV, APETREI C, Disposable biosensors based on carbonaceous screen-printed electrodes and diamine oxidase, Curr. Opin. Chem. Biol., 24, S65 (2013). 20. MACIEJEWSKA J, PISAREK K, BARTOSIEWICZ I, KRYSINSKI P, JACKOWSKA K, BIEGUNSKI AT, Selective detection of dopamine on poly(indole-5-carboxylic acid)/tyrosinase electrode, Electrochim. Acta, 56, 3700-3706 (2011). 21. ARDUINI F, DI GIORGIO F, AMINE A, CATALDO F, MOSCONE D, PALLESCHI G, Electroanalytical Characterization of Carbon Black Nanomaterial Paste Electrode: Development of Highly Sensitive Tyrosinase Biosensor for Catechol Detection, Anal. Lett., 43, 1688-1702 (2010). 22. LIU X, ZHANG J, YAN R, ZHANG Q, LIU X, Preparation of graphene nanoplatelet-titanate nanotube composite and its advantages over the two single components as biosensor immobilization materials, Biosens. Bioelectron., 51, 76-81 (2014). 23. WANG HS, HUANG DQ, LIU RM, Study on the electrochemical behavior of epinephrine at a poly(3methylthiophene)-modified glassy carbon electrode, J. Electroanal. Chem., 570, 83-90 (2004). 24. SVITEL J, MIERTUS S, Development of Tyrosinase-Based Biosensor and Its Application for Monitoring of Bioremediation of Phenol and Phenolic Compounds, Environ. Sci. Technol., 32, 828-832 (1998). 25. LUPU S, LETE C, BALAURE PC, CAVAL DI, MIHAILCIUC C, LAKARD B, HIHN JY, DEL CAMPO FJ, Development of Amperometric Biosensors Based on Nanostructured TyrosinaseConducting Polymer Composite Electrodes, Sensors, 13, 6759-6774 (2013). 26. KURGANOV BI, LOBANOVA AV, BORISOV IA, RESHETILOV AN, Criterion for Hill equation validity for description of biosensor calibration curves, Anal. Chim. Acta, 427, 11-19 (2001). 27. BARD AJ, FAULKNER LR, Electrochemical Methods, John Wiley and Sons, New York, 2001. 28. SANZ VC, MENA ML, GONZÁLEZ-CORTÉS A, YÁÑEZ-SEDEÑO P, PINGARRÓN JM, Development of a tyrosinase biosensor based on gold nanoparticles-modified glassy carbon electrodes. Application to the measurement of a bioelectrochemical polyphenols index in wines, Anal. Chim. Acta, 528, 1-8 (2005). 29. Farmacopeea Romana, Editia a X-a, Ed. Medicala, 1993, pp. 381, 392. 30. APETREI IM, RODRIGUEZ-MENDEZ ML, APETREI C, DE SAJA JA, Enzyme sensor based on carbon nanotubes/cobalt(II) phthalocyanine and tyrosinase used in pharmaceutical analysis, Sens. Actuators B, 177, 138-144 (2013). 287 288 289 290 291 292 293 294 295 296 297 298 299 300 301 302 303 304 305 306 307 308 309 310 311 312 313 314 315 316 317 318 319 320 321 322 323 324 325 326 327 328 329 330 331 332 333 334 335 336 337 338 339 340 341