Measuring Matter: Cornell Notes on Mass, Volume, Density
advertisement

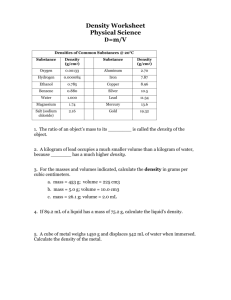
Cornell Notes Paper Topic: Measuring Matter Date: 10/29/14 Source: ItC, 1.3, 14-19 Main Topics Detailed Notes What units are used to express mass and volume? Weight – a measure of the force of gravity on an object (unit used his newtons) o weight changes based on the planet – different gravitational pull equals different weight mass – the amount of matter in an object o mass doesn't change wherever you are – the amount of matter in an object remains constant o mass is measured in kilograms (kg) – grams are used for smaller measures – there are 1000 g in a kilogram volume – the amount of space matter occupies – all matter takes up space – solids, liquids, and gases o liquid volume is measured in Litres (L) o smaller liquid volumes are measured in milliliters (mL) o solid volume is measured in cubic centimeters (cm3) o 1 mL = 1 cm³ finding the volume of a rectangular object uses the formula: length x width x height finding the volume of an irregular object – submerge the object in a graduated cylinder – liquid level will rise by an amount equal to the volume of the object in milliliters How is density determined? Density – the measure of the mass in a given volume expressed as the number of grams in 1 cm³ (g/cm3) o density of water is 1 g/cm³ – can also be expressed as 1 g/mL KEY CONCEPT – density of material can be found by dividing its mass by its volume o density = mass/volume objects with density greater than water will sink – if they have less density than water, they will float Cornell Notes Paper Topic: Measuring Matter Date: 10/29/14 Source: ItC, 1.3, 14-19 Main Topics Detailed Notes Using density If you know the density of a substance you can use it to identify an unknown substance measure the mass and volume of the substance that you don't know – if the densities match, you have the same substance. If they differ, they are different substances. Cornell Notes Paper Topic: Date: Source: Main Topics Detailed Notes Sample Sample