research_paper_a.richins
advertisement

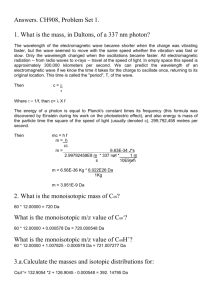
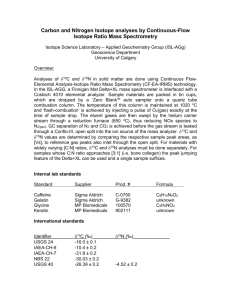
Isotope Fractionation in Plants and Local Plant Isoscapes at Sevilleta Wildlife Refuge Allyson Richins, REU 2013 Abstract Predation plays a dramatic ecological role at Sevilleta, directly resulting in the cycling of nutrients from producers to primary and secondary consumers, and indirectly resulting in the cycling of nutrients to scavengers, detrivores, and ultimately the soil (Schmitz et al). This project set a foundation for future food web research by examining isotopic fractionation in a diversity of plant species between two distinct microhabitats, grassland and shrubland. Fractionation data was compared between different microhabitats, different years, and different species at a particular location. Results indicated statistical significance in 13C and 15N between different species across each array, and significance for 13C values between different microhabitats for most plants. 13C values for particular species had a much lower range than the values for 15N. An isoscape map was also constructed to better visualize the spread of flora at the different habitats. In the future, the isotopic data collected this summer will be compared to isotopic analysis of insect and small mammal tissues to determine animal diets, and to determine whether these organisms are generalists, or C3 or C4 specialists. Introduction Sevilleta Wildlife refuge is home to a unique diversity of life. These organisms interact in every conceivable way, both helpful and harmful. Mutualism occurs frequently when mycorrhizal fungi fix nitrogen in the soil for the absorption of plants, while the roots of the plant provide carbohydrates necessary for the fungi’s survival (van der Heijden et al 2010). At the same time, competition for prey items between predators such as snakes and birds of prey has a negative effect on both organisms. Predation probably plays the most dramatic role across the ecosystem, directly resulting in the cycling of nutrients from producers to primary and secondary consumers, and indirectly resulting in the cycling of nutrients to scavengers, detrivores, and ultimately the soil (Schmitz et al 2010). Food webs provide visual simulations of predation across a community. Their construction often relies on visual observation of organisms and scat analysis. Drawbacks to these methods include time intensive research in often-unreliable conditions, and very shortterm information about diet, respectively (Kaufman & Michener 2008). Isotopic analysis is a method to determine animal diets over a long span of time, and produces relatively prompt results (Kaufman & Michener 2008). This technique of determining animal food sources can reveal dietary patterns and is widely used to construct food webs (Kaufman & Michener 2008). In nature, multiple isotopes of any element exist in any given substance. The ratio of heavier to lighter isotopes is known as the ‘R Value.” Discrepancies in R-values are generally very small, and so they are expressed in a “∂ value,” defined as: (Oleary 1988) Whenever a chemical reaction occurs, or an element changes chemical forms, isotope fractionation occurs, a phenomenon whereby one isotope is enriched relative to another. This enrichment occurs due to mass differences between heavier and lighter isotopes. As a result of this enrichment, heavier isotopes tend to concentrate in certain substances, for instance, plant tissues (Oleary 1988). During photosynthesis, plants discriminate against 13C over 12C, 15N over 14N, and 2H over 1H. Plants with different photosynthetic pathways fractionate isotopes in different ratios. C3 plants experience lower fractionation of carbon compared to C4 plants, and thus accumulate less 13C in their lifespans. C3 plants have a lower ∂C value than C4 plants. CAM plants experience ∂C values lower than C3 or C4 plants. Isotope analysis can be used to determine a plants’ photosynthetic pathway and gives information about water use efficiency (Oleary 1988). This project examined isotopic fractionation in plants both across different microhabitats and across the same microhabitat. As such, isoscapes were created to illustrate variation in vegetation at a localized level. Isoscapes, also known as isotopic landscapes, are an application of isotope analysis that allow for the visualization of plant types and fractionation data over a habitat scale. They have been used to monitor changes in plant distribution and nutrient availability over long time scales. This project also compared recent fractionation data to past records. In 2005 and 2006, Dr. Blair Wolf conducted isotopic analysis on a diversity of plants at the Five Points sites. 2005 was an average rainfall year, while 2006 experienced no winter precipitation and doubled monsoonal rainfall. As New Mexico experienced a dramatic drought during 2011-2012, the plant fractionation data from summer 2013 represents plant vitality after a two-year drought. Methods Research was conducted at two sites, the first (Site 1) situated in mixed grassland/shrubland, and the second site (Site 2) located in a grassland/shrubland transition zone. Half of Site 2 is composed of a grassland ecosystem, while the other half is composed of mixed grassland/shrubland. The shrubland ecosystem primarily consists of creosote (Larrea tridentata) and contains large areas of bare ground. The grassland ecosystem is dominated by black grama grass (Bouteloua eriopoda) but also contains ring Muhly grass (Muhlenbergia torreyi), blue grama grass (Bouteloua gracilis), and scattered fluff grass (Dasyochloa pulchella). Both sites are located in the Five Points area, southeast of Black Butte. Samples were collected across circular trapping arrays that were previously established by Dr. Blair Wolf in 2005. These arrays are circular trapping webs, containing 145 Sherman traps arranged in 12 spokes. Plant samples were collected in a 95 cm radius around every other trap at the north, south, east, and west transects. One plant of each species within the radius was selected and a 2-inch long sample of the most viable (living) portion of the plant was collected. If multiple plants of a particular species were present, the most viable specimen was selected. Additionally, rarer plants, not found along the transect lines were identified in the areas between transects and sampled. These samples were placed into coin envelopes and later transferred into a drying oven. The plants were dried until all moisture evaporated from them. One fragment of 0.2-0.3 mg and one sample of 2.0-3.0 mg of each plant sample were collected. The smaller fragments were placed into silver capsules and the larger fragments were placed into tin capsules. The capsules were then placed into a 96-well plate. The samples were then analyzed for stable isotope fractionation of carbon, hydrogen, and nitrogen. The data from this analysis was analyzed for statistical significance using a one-column ANOVA. Statistically significant differences in past to present isotope fractionation data were also analyzed using ANOVA. All statistical analysis was conducted using a 95% confidence level. Isoscape maps were created using convex hull mapping of unique microhabitats across the arrays. These unique areas included areas of high creosote density, rocky areas, grassy areas, streambeds, and areas with high concentrations of unique plants (juniper, yucca). The coordinates were input into a Garmin GPS unit and later put into an ArcMap program, so polygons defining these spaces could be constructed. These polygons were projected onto a Bing satellite map of the 5 points area. Buffering zones around polygons were estimated using an average of three measurements of vegetation concentration around each polygon. Results Comparison of plant diversity between each array showed that some species were similar across both arrays, while some were unique to particular locations. As shown in figure 1, shrubs such as creosote and sagebrush, and associated plants such as bush Muhly grass were found in higher concentrations at Site 1, while a wider range of grasses and small forbs were identified at Site 2. Overall, higher concentrations of C3 plants were present at Site 1 in comparison to higher concentrations of C4 plants at Site 2. Tables 1 and 2 contain ANOVA results for 13C and 15N. Each unique letter corresponds to a statistically significant species, and different species that were signified by the same letter(s) are not statistically significant. Overall, the diversity of plants was shown to be statistically significant, with an overall P value of less than .0001 for both 13C and 15N at each site. The 13C values for a given plant species tended to be fairly consistent, while a wider range of 15N values were observed for the species sampled. C3 and C4 plants exhibited statistically significant differences in 13C, while CAM plants did not exhibit statistically significant differences from C3, but did exhibit significant differences from c4. This trend was shown to be statistically significant for both Site 1 and Site 2. Across both sites, d13Cvalues for different photosynthetic pathways were relatively consistent. In comparison, wide variability in 15N values was observed among distinct photosynthetic pathways. Figures 2 and 3 provide visualization of these trends. Assessment of past and present isotopic values revealed little overall variation in 13C or d15n, as demonstrated in figure 4. Comparison of the standard deviations of the data also showed consistently wider variations in d15n values in plants as compared to a relatively narrow range of values for 13C. A comparison of fractionation values between the different grids revealed statistically greater d13C values at Grid 1 over Grid 2 for creosote, with a P value of 0.028, prickly pear, with a P value of 0.010, and an average of dagger and cane chollas, with a P value of 0.024, as indicated by Figure 5. A statically lower 13C value at Grid 1 over Grid 2 was observed for an average of black and blue grama grass, with a P value of 0.015. No statistically significant trend for 13C existed for dogweed between the two different grids, with a P value of 0.273. Additionally, no statistically significant trends were found for d15n between the different grids for any of the plants analyzed. Figure 6 depicts an isoscape for Grid 1. This map shows considerable changes in vegetation across a limited microhabitat. Discussion This project revealed the accuracy with which 13C values can be used to determine photosynthetic pathway used by a particular plant species. C4 and C4 plants were very distinct from one another. The lower fractionation in C3 plants than C4 plants supports previous data regarding the efficiency of the different photosynthetic pathways (Gowick & Westhoff 2011). C3 plants experience a process known as photorespiration during CO2 fixation. This process results in the sometimes binding of oxygen to the plant’s CO2 fixing compounds. As a result, the C3 pathway is less efficient at fixing CO2 than the C4 pathway (Oleary 1988). The wide range of 15N values across both similar and different photosynthetic pathways suggests is not related to nitrogen acquisition. correlates to the relative successes of different plant species as nitrogen fixers, or as rhizomatous plants (van der Heijden et al 2010). For example, Pleurophis jamesii (Galleta grass) has mycorrhizal associations with mutualistic soil fungi (Simonen 2011). As such, the species exhibited 15N values lower than nitrogen gas in the atmosphere. Significant differences between the two different grids existed for 13C for most species. Likely, this difference was due to the times that this data was collected. Plant samples were collected from Grid 1 during an intense dry period, and samples were collected from Grid 2 two weeks later, after monsoon rainfall had begun. The added moisture may have caused mobilization of nutrients in the soil, allowing for increased uptake. This pulse of rain also promoted increased growth, which may explain the increased 13C values in the second array. Statistics could not be run on the past to present data, due to the lack of replication from the past data. Some of the species had a sample size of one, and so meaningful statistical analysis could not be run. In the future, the isotopic data collected this summer will be compared to isotopic analysis of insect and small mammal tissues to determine animal diets, and to determine whether these organisms are generalists, or C3 or C4 specialists. The future of this project should yield exciting results that can be used to construct food webs at Sevilleta. CITATIONS 1) O’Leary, M. (1988). Carbon Isotopes in Photosynthesis. Bioscience. Vol 38: 5: 328-336 2) Schmitz, OJ., Hawlena, D., Trussell, G. C. (2010). Predator control of ecosystem nutrient dynamics. School of Forestry and Environmental Studies. Yale University, New Haven, CT 06511, USA. 3) Simonin, Kevin A. (2011). Pleuraphis jamesii. Fire Effects Information System. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). 4) Gowik, U. & Westhoff, P. (2010) The Path from C3 to C4 Photosynthesis. Institut für Entwicklungs und Molekularbiologie der Pflanzen. Heinrich-Heine-Universität, 40225 Duesseldorf, Germany 5) van der Heijden, M. G. A., Klironomos, J. N., Ursic M. Moutoglis, P., Streitwolf-Engel, R., Boller, P., Wiemken, A. & Sanders, I. R. (2010) “Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity.” Ecol Lett. Vol 10:1199-209. 6) Kaufman & Michener. (2008). Chapter 9. Stable Isotopes as tracers in marine food webs: An update. Pg 238-240