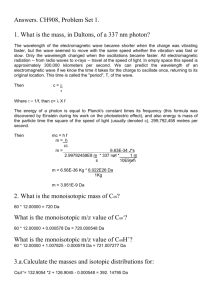
Thesis: 13C increases (becomes less negative) depth
advertisement

Thesis: 13C increases (becomes less negative) depth. Reasons - Wynn Mixing Of atmospheric CO2 at time of entry to soil pores (date) Physical mixing of soil organic matter (SOM) Preferential decomposition Kinetic fractionation Reasons – Ehleringer 2000 1. Suess effect – a. Older, deeper SOM originated when atmospheric 13C was more positive (CO2 was heavier) b. From 1744 to 1993, difference in 13C app -1.3 ‰ c. Typical soil profile differences = 3 ‰ 2. Microbial fractionation during decomposition a. microbes choose lighter C b. frequently use Rayleigh distillation analyses c. no direct evidence for this 3. Preferential microbial decomposition a. Plant compounds vary up to 10 ‰ b. Problem: lignin:C increases with depth and as SOM decreases c. Lignins lighter than leaf values d. So trend in opposite directions 4. Soil carbon mixing – a. Microbial and fungal C residue (Ehleringer, 2000, p 418) – b. some of the carbon incorporated into SOM by these critters has an atmospheric, not SOM source. c. Atmospheric C is heavier. The CO2 from atmos in the soil is 4.4 ‰ heavier than CO2 metabolized by decomposition (Wedin, 1995) Wedin 1995 studied lignin in grasses. Most prior decomposition studies had been in forests, where lignin is more overwhelming. Order of decay of compounds Melillo 1989 1. loss of C fractions depleted in 13C (p 192) a. tannins b. non-polar components c. water-soluble compounds d. lignin, also depleted in 13C is conserved 2. Cellulose – C fraction enriched in 13C 3. Lignin a. Recalcitrant b. Can be enhanced by addition of simple sugars c. N may slow lignin decay (Fenn etal 1981, Keyser etal 1978) – not proven Controls on decay (Melillo, et al, 1989) 1. Temperature 2. Moisture 3. Soil texture 4. Availability of labile C and N 5. 13C ‰ Wynn et al, 2006 Try to determine which process controls 13C depth profile Fig. 2. Soil organic carbon concentration and isotope depth profiles of 4 slope settings from control (Goodwin Creek) and cropland (Nelson Farm) sites. Depth is plotted as depth below the mineral surface. Top row shows carbon concentration profiles, C(z), fit to Eq. (7) in the text (data from replicate profiles: closed circles, solid line=bulk SOC control site; open circles, dashed line=bulk SOC cropland site; parameters of fit in Table 1). Second row shows stable carbon isotopic composition, d13C, of solid and gas phase. Crosses show d13C of incubated samples with horizontal lines indicating discrimination to time series of stable isotope measurements of CO2 samples produced by incubation (black numbers=respired CO2 control sites; gray numbers=respired CO2 cropland sites). These time series data are shown in more detail in Fig. 6. Third row shows radiocarbon composition, FM, of solid phase bulk SOM. Crosses show FM of incubated samples prior to incubation for upper and lower slope settings. Wynn fig 2 – undisturbed site = (black dots) Disturbed (agricultural) site = open dots “Kink” in the Cz curve reflects root depth or productivity zone Rayleigh distillation. This is an exponential function that describes the progressive partitioning of (in this case) heavy isotopes into the water reservoir as it diminishes in size: R = Ro f (–1) http://wwwrcamnl.wr.usgs.gov/isoig/isopubs/itchch2.html#2.3.3 2.3.3 The Rayleigh equation The isotopic literature abounds with different approximations of the Rayleigh equations, including the three equations below. These equations are so-named because the original equation was derived by Lord Rayleigh (pronounced "raylee") for the case of fractional distillation of mixed liquids. This is an exponential relation that describes the partitioning of isotopes between two reservoirs as one reservoir decreases in size. The equations can be used to describe an isotope fractionation process if: (1) material is continuously removed from a mixed system containing molecules of two or more isotopic species (e.g., water with 18O and 16O, or sulfate with 34S and 32S), (2) the fractionation accompanying the removal process at any instance is described by the fractionation factor , and (3) does not change during the process. Under these conditions, the evolution of the isotopic composition in the residual (reactant) material is described by: (R / Rº) = (X1 / X1º) -1 (Eq. 2.11) where R = ratio of the isotopes (e.g., 18O/16O) in the reactant, Rº = initial ratio, Xl = the concentration or amount of the more abundant (lighter) isotope (e.g.,16O), and X1º = initial concentration. Because the concentration of Xl >> Xh , Xl is approximately equal to the amount of original material in the phase. Hence, if ƒ = Xl/X1º = fraction of material remaining, then: R = Rº ƒ(-1) . (Eq. 2.12) Another form of the equation in -units is: º ƒ(-1) (Eq. 2.13) which is valid for values near 1, º values near 0, and values less than about 10. In a strict sense, the term "Rayleigh fractionation" should only be used for chemically open systems where the isotopic species removed at every instant were in thermodynamic and isotopic equilibrium with those remaining in the system at the moment of removal. Furthermore, such an "ideal" Rayleigh distillation is one where the reactant reservoir is finite and well mixed, and does not re-react with the product (Clark and Fritz, 1997). However, the term "Rayleigh fractionation" is commonly applied to equilibrium closed systems and kinetic fractionations as well (as described below) because the situations may be computationally identical. Rayleigh distillation equation used by Wynn, 2006 13Cf 1 1000 1 F 13Ci 1 1 e t 1 1 1000 e 1t 1 t F 13Cf 13Ci α e t fraction of remaining soil organic matter (SOC) – approximated by the calculated value of fSOC isotopic composition of SOC when sampled isotopic composition of input from biomass fractionation factor between SOC and respired CO2 efficiency of microbial assimilation fraction of assimilated carbon retained by a stabilized pool of SOM assumptions by Wynn etal 1. Open system a. All components decompose b. Contribute to soil-respired CO2 at same rate with depth 2. FSOC fSOC Rayleigh distillation is used to describe the kinetic fractionation Fig. 3. Regression analysis of the degree of decomposition of soil organic carbon ( fSOC) to its stable isotopic composition (d13CSOC) for the various erosional regimes of the MBPC control sites (non-agricultural, Goodwin Creek). The Rayleigh distillation relationship following Eq. (11) is shown prior to and following subtraction of a linear fSOC to d13CSOC relationship (a mixing line), as is discussed in the text. Parameters of fit are found in Table 2. Wynn fig 3 – undisturbed site - as decomposition age increases, 13C increases – enriches Fig. 4. Regression analysis of the degree of decomposition of soil organic carbon to its stable isotopic composition for the various erosional regimes of the MBPC cropland sites (Nelson Farm). Distillation relationship for the corresponding control sites (Goodwin Creek) from Fig. 3 are shown in gray for comparison. Only the valley profile shows a significant correlation to the distillation model although it is different from the valley setting at the control site. Uncorrected and corrected relationships for this profile are shown in black lines as in Fig. 3. Wynn fig 4 – disturbed site – doesn’t conform as well to the model – Why not? Fig. 9. Model of agricultural effects on the relationship of SOC concentration to isotopic composition (plots of d13CSOC to fSOC as shown in Figs. 3 and 4 for control and cropland sites). A. Natural trend resulting from kinetic fractionation during decomposition (following the Rayleigh distillation equation). B. Installation of C4 crops increases d13CSOC of surface materials in proportion to new SOC production. C. Enhanced decomposition of labile SOM due to cropping residually accumulates more decomposed and 13C-distilled SOC, particularly at the surface. D. Erosion of surface horizons (without SOC replacement) removes the upper portion of the line in A. Because fSOC is normalized to a value of 1 at the new surface, the shortened line is also shifted upward. E. Regeneration of a new SOC concentration profile over an eroded profile mixes new labile 13C-depleted SOC, diluting the decomposed and 13C-distilled SOC from the previously eroded soil. Because SOC production is nonlinear with depth, the lowermost samples are most diluted while surface samples are less diluted, producing a curved relationship. Wynn fig 9 – various reasons that disturbed land might not conform to nice regression curve in fig 3 A – natural B – introduce C4 plants, enriched in 13C C – Cropping – removes new, low 13C material, leading to surface enrichment D – Erosion – removes upper layer, moving the whole curve up E – Reintroduce soil organic carbon (better management practices) – reverses the trends in C, D, and E