Chem: PS Ticket to Retake
advertisement

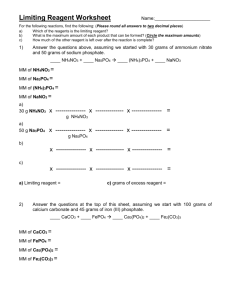
Chemistry Problem Set: Ticket to Retake Limiting Reagent Block: ____ Name: ________________ There will be a short limited retake of the limiting reagents quiz on Thursday Jan. 31st (available during academic lab and after school). You must let me know which time you will be attending. If you plan on taking the retake, you must: 1) correctly redo (and bring to the retake) PS D5 _______ correctly redo (and bring to the retake) PS D6 _______ correctly complete this problem set (and bring it to the retake) When zinc metal is placed in a solution of silver nitrate a reaction yielding silver metal and zinc nitrate occurs. a) Write the balanced chemical reaction. b) 2) _______ Suppose you had mass of 2.00 g of zinc metal and a solution containing 2.50 g of silver nitrate. i) What is the limiting reagent? ii) What mass of silver is produced? iii) What mass of zinc nitrate is produced? iv) What mass (in grams) of the excess reagent remains after the reaction has completed? Sodium hydroxide reacts with carbon dioxide to form sodium carbonate and water. a) Write the balanced chemical reaction. b) Suppose you had 1.85 mol of sodium hydroxide and 1.00 mol of carbon dioxide. i) What is the limiting reagent? ii) How many moles of the excess reagent remain? iii) How many moles of sodium carbonate are formed? 3) 4) Another reaction of ammonia and oxygen produces nitrogen oxide and water. a) Write the balanced chemical reaction. b) Suppose you had 1.5 g of ammonia and 2.75 g of oxygen. i) What is the limiting reagent? ii) How many grams of the excess reagent remain? iii) How many grams of nitrogen oxide and water are formed? Solid calcium reacts with nitrogen gas to yield calcium nitride. a) Write the balanced chemical reaction. b) Suppose you had 50 grams of calcium and 50.0 grams of nitrogen gas. i) What is the limiting reagent? ii) How many grams of the excess reagent remain? iii) How many grams of calcium nitride are formed? Some of the answers IRO (more later): 61.7 0.925 1.39 1.52 0.329