Limiting Reagent Worksheet
advertisement
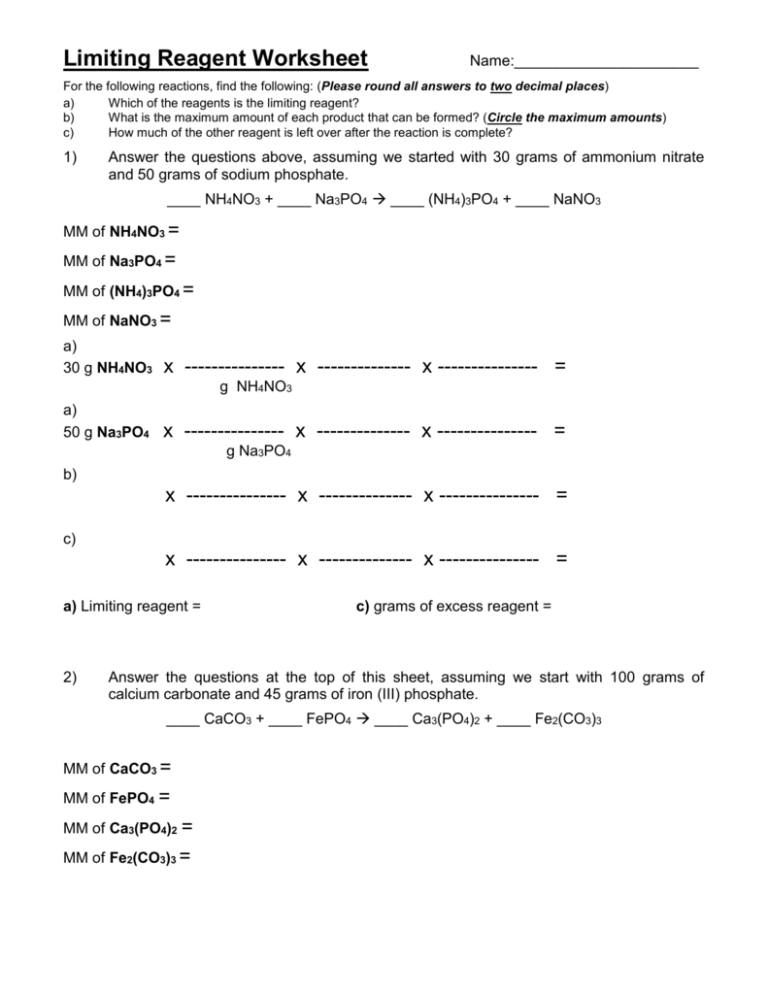
Limiting Reagent Worksheet Name:______________________ For the following reactions, find the following: (Please round all answers to two decimal places) a) Which of the reagents is the limiting reagent? b) What is the maximum amount of each product that can be formed? (Circle the maximum amounts) c) How much of the other reagent is left over after the reaction is complete? 1) Answer the questions above, assuming we started with 30 grams of ammonium nitrate and 50 grams of sodium phosphate. ____ NH4NO3 + ____ Na3PO4 ____ (NH4)3PO4 + ____ NaNO3 MM of NH4NO3 = MM of Na3PO4 = MM of (NH4)3PO4 = MM of NaNO3 = a) 30 g NH4NO3 x --------------- x -------------- x --------------- = g NH4NO3 a) 50 g Na3PO4 x --------------- x -------------- x --------------- = g Na3PO4 b) x --------------- x -------------- x --------------- = c) x --------------- x -------------- x --------------- = a) Limiting reagent = 2) c) grams of excess reagent = Answer the questions at the top of this sheet, assuming we start with 100 grams of calcium carbonate and 45 grams of iron (III) phosphate. ____ CaCO3 + ____ FePO4 ____ Ca3(PO4)2 + ____ Fe2(CO3)3 MM of CaCO3 = MM of FePO4 = MM of Ca3(PO4)2 = MM of Fe2(CO3)3 = a) 100 g CaCO3 x --------------- x -------------- x --------------- = a) 45 g x --------------- x -------------- x --------------- = FePO4 b) x --------------- x -------------- x --------------- = c) x --------------- x -------------- x --------------- = a) Limiting reagent = 3) c) grams of excess reagent = Answer the questions at the top of this sheet, assuming we start with 74.58 grams of sodium hydroxide and 18.94 grams of sulfuric acid? ____ NaOH + ____ H2SO4 ____ H2O + ____ Na2SO4 = = = = a) x --------------- x -------------- x --------------- = a) x --------------- x -------------- x --------------- = b) x --------------- x -------------- x --------------- = c) x --------------- x -------------- x --------------- = a) Limiting reagent = 4) c) grams of excess reagent = On a separate sheet of paper - Answer the questions at the top of this sheet, assuming we start with 43.51 grams of lithium nitrate and 5.23 grams of lead (IV) sulfate? ____ Pb(SO4)2 + ____ LiNO3 ____ Pb(NO3)4 + ____ Li2SO4








