Recommended Template for NSAG Patient Care Pathways
advertisement

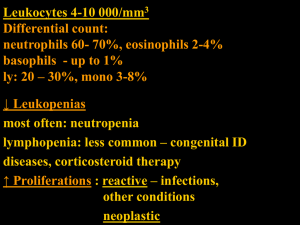
Acute Myeloid Leukaemia (AML) Haematological Pathway for South Wales Cancer Network Document Control Sheet Organisation Specialty/Project Document Title Document Number Version 2.0 Approved by South Wales Haematology Cancer Network Group South Wales Cancer Network Haematological Site Specific Group Acute Myeloid Leukaemia Haematological Pathway 05/006 Author/s Dr S Knapper Dr J Kell Dr C Alvares Dr U Mohite Approval date Ratified by 19/03/15 19/03/16 Dr C Fegan Dr W Ingram Date of next review Acute Myeloid Leukaemia NSAG Patient Care Pathway Demographics - Approximately 100 new cases in Wales per year (2,500 in UK). Median age is 68. Incidence rises from ~1-2 per 100,000 <45yrs to 10-15 per 100,000 >70yrs - This pathway covers patients with a diagnosis of AML aged 16 years upwards Diagnosis - Confirmed by the presence of ≥20% myeloid blast cells in the bone marrow - May alternatively be made by demonstrating a recurrent cytogenetic abnormality: t(8;21), inv(16), t(16;16), t(15;17) irrespective of blast count Required tests: - Full blood count with differential white blood count - Blood film examination - Bone marrow aspirate: (trephine optional – but essential if ‘dry tap’) Morphology Immunophenotypic characterisation of blast population Cytogenetics (or, if fails, FISH for recurrent abnormalities) - Coagulation screen (including fibrinogen level) - Renal and liver chemistry Additional investigations: - Molecular genetics: PML-RARA, RUNX1-RUNX1T1, CBFB-MYH11 consider FLT3, NPM1 mutation screening - HLA-typing and CMV serology should be performed at the earliest available opportunity (potential allogeneic stem cell transplant candidates only) - If CNS involvement is suspected: lumbar puncture and CSF cytospin - Echocardiogram (prior to intensive therapy if ≥60yrs / cardiac comorbidity) Staging and Prognostication - There is no formal ‘staging’ system in AML - Patient-related factors include age, performance status, co-morbidities - AML-related factors: presenting WBC primary/secondary disease cytogenetic risk group FLT3/NPM1 mutation status Information Required at MDT At the time of initial MDT discussion: - Blood and bone marrow morphology, immunophenotyping - Initial treatment plan, including clinical trial eligibility / entry Acute Myeloid Leukaemia (AML) 1 Patients aged between 16-24yrs should be referred to the appropriate Teenage / Young Adult (TYA) team and discussed at the TYA MDT. The specialist diagnostic information required for precise disease characterisation (including cytogenetics and molecular studies) will generally be unavailable at the initial MDT discussion. This information should be added at a follow-up MDT discussion 2-4 weeks later in order to accurately document: - Diagnosis according to Revised WHO classification (2008) - ICD10 v4 and morphology codes - Suitable minimal residual disease (MRD) marker, if identified - Appropriateness of allogeneic stem cell transplant in first complete remission (for patients considered likely to be candidates for allograft, the Cardiff BMT team should be informed as soon as possible following this discussion) Indications to Treat - AML is frequently an emergency presentation and the overwhelming majority of cases require a prompt treatment decision and initiation of active therapy. - In older patients AML may evolve more slowly, often from antecedent MDS allowing a brief period of initial observation, during which fitness for therapy can be assessed and prognostic results (including cytogenetics) obtained. Treatment General The management of all new AML cases should be discussed by the MDT. Given the emergency presentation of many patients, initial treatment will often necessarily need to be instituted before the first formal MDT review. Patients should be offered clinical trial entry where possible. Typically a range of studies will be available including intensive and non-intensive therapies, and trials for those with newly-diagnosed or relapsed / refractory disease. Supportive treatment: - Patients with excessive leucocytosis and accompanying clinical evidence of leucostasis may require emergency leukapheresis before commencing chemotherapy. This will only apply to a small percentage of cases. Additionally, oral hydroxycarbamide (2-3g/day) should be used for initial cytoreduction prior to the commencement of formal chemotherapy. - Recombinant uric oxidase (rasburicase) should be used prior to initiation of chemotherapy in patients with WBC>100x109/l or in those with metabolic disturbances which increase the risk of acute tumour lysis syndrome. - Blood and platelet transfusions should be used according to the FBC and clinical situation (also see specific guidance for APL). Irradiated cellular blood products should follow purine analogue-containing chemotherapy regimens. - Antimicrobial prophylaxis should be given according to local guidelines. Acute Myeloid Leukaemia (AML) 2 Intensive therapy: - Intensive chemotherapy is considered ‘standard of care’ for patients <60yrs, as well as those >60yrs with good performance status. - Where possible, patients should be treated within the age-appropriate NCRI clinical trial schedule (no current trial for under 60 years although AML19 will open during 2015; AML18 open for patients aged over 60 years suitable for intensive treatment). - Patients who are ineligible or unwilling to participate in clinical studies should be offered standard induction therapy including an anthracycline and cytarabine (typically DA3+10). There is no consensus on a single best post-remission schedule: generally patients will receive a further cycle of DA (3+8) followed, in younger adults in whom allogeneic SCT is first remission is considered inappropriate, by 2 cycles of consolidation with a high dose cytarabine-containing regimen. - Allogeneic stem cell transplant in first complete remission should be considered in patients with adverse and intermediate-risk disease and a suitable HLA-matched donor. It is not justified in favourable-risk patients. - Patients identified as potential allogeneic transplant recipients should be discussed with the BMT team in Cardiff at the earliest available opportunity Relapsed / Refractory AML: - Patients failing to respond to 1-2 cycles of induction treatment are considered ‘refractory’ and have a poor prognosis. For relapsing patients, a longer first remission is associated with a greater chance of subsequent treatment success. - Carefully selected patients with relapsed / refractory disease may be offered further intensive chemotherapy, usually with a view to allogeneic stem cell transplantation if a second remission is attained. If available, patients should be treated within a suitable clinical trial protocol for relapsed patients. - Patients unsuited to further intensive chemotherapy should be offered less-toxic non-intensive (palliative) treatment, again within a suitable clinical trial protocol where possible. Non-intensive therapy: - The majority of patients >70yrs and younger patients with significant comorbidity will receive non-intensive (palliative) chemotherapy. - Where possible, patients should be treated within an appropriate clinical trial. Current local trial options include the NCRI LI-1 study (randomised comparison of low dose cytarabine with a ‘rolling programme’ of novel non-intensive chemotherapy approaches) and RAVVA (randomised comparison of azacitidine with or without the histone deacetylase inhibitor vorinostat). - Low dose cytarabine remains standard of care for patients not entering clinical trials. - The hypomethylating agent azacitidine may be considered in patients with 20-30% bone marrow blasts at presentation, and is NICE-approved for this indication. Acute Myeloid Leukaemia (AML) 3 - Patients who are not felt able to tolerate non-intensive chemotherapy should be given best supportive care: transfusion support and hydroxycarbamide as required to control the white cell count. Acute Promyelocytic Leukaemia (APL): - The presentation of APL is a haematological emergency due to the high early mortality rate as a consequence of associated coagulopathy - All trans-retinoic acid (ATRA) should be started as soon as the diagnosis is suspected (45mg/m2/day in divided doses). For patients presenting to outlying DGHs, ATRA should be commenced before patient transfer. - FBC and coagulation screen should be checked at least twice daily during the initial phase of treatment. The platelet count should be maintained at >50x109/l and FFP (and/or cryoprecipitate/ fibrinogen concentrates) used to normalise coagulation times and maintain fibrinogen level close to 2g/l. - Diagnosis should be confirmed promptly by demonstration of the PML/RARα fusion (initially by FISH, then confirmed by molecular studies.) - There is no currently-open clinical trial available for patients with APL, but the AML19 is likely to include a research question for APL patients and will open during 2015 - Patients deemed suitable for intensive therapy should be treated with ATRA and anthracycline-based induction and consolidation therapy (AIDA schedule) - Arsenic trioxide (ATO), also used with ATRA, should be considered in patients deemed unsuitable for anthracycline-based therapy. - Minimal residual disease assessment (see below) should be used to guide the need for further therapy. In the event of relapse (full relapse, or confirmed molecular relapse with rising PML/RARα transcript levels) ATO should be used, and consolidation with autologous transplant considered. Monitoring - Patients receiving active therapy require close monitoring with serial blood counts and further bone marrow aspirates to assess and monitor remission status. - Following conclusion of active treatment patients should be followed up clinically and with repeated blood count evaluation. - In APL, the value of molecular monitoring (Q-PCR to measure PML/RARα transcript level) in order to detect relapse at an early stage and intervene pre-emptively is established. APL patients should be offered 3-monthly bone marrow monitoring for 2-3 years following completion of active treatment. - For non-APL patients, serial bone marrow examinations in remission are of uncertain value: the use of MRD monitoring in this context is currently being evaluated within clinical studies (AML17, and to continue within AML19). - AML patients receiving supportive treatment only will require individualised monitoring depending on their transfusion and other requirements Discharge - Discharge may be offered to patients who have achieved stable longstanding complete remission (usually >5 years from completion of active therapy). Acute Myeloid Leukaemia (AML) 4 References Diagnosis and management of AML in adults: recommendation from an international expert panel, on behalf of the European LeukemiaNet. (Dohner et al. Blood 2010; 115: 453-473) BCSH Guidelines on the management of AML in adults. (Milligan et al. BJH 2006; 135:350-474) WHO classification of tumours of hematopoietic and lymphoid tissues. (edited by Swerdlow et al. 2008) Z:\ststorage2\South Wales Cancer Network\SWCN\Tumour Site\Haematological\Pathways\006 AML revised (2 2015).docx Acute Myeloid Leukaemia (AML) 5