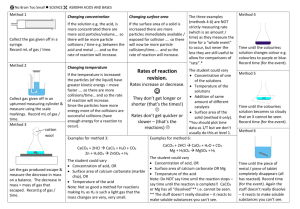
Rate of reaction increases b/c more collisions
advertisement

Key HOMEWORK for Chapter 13: Chemical Equilibrium 13.1 Rates of Reactions 1. (Read pgs. 406 - 412 in the chemistry textbook) What is the Collision Theory? A theory that says that a reaction will only take place when the reacting molecules a) collide, and have b) the proper orientation with c) sufficient energy 2. What is activation energy(Ea)? the minimum energy required to initiate a chemical reaction; it is the amount of energy required to break bonds between the atoms of the colliding reactant molecules 3. What is an energy diagram? a graph of the progress of a chemical reaction vs. the total energy of the system Chemical reaction does not start until the reactants gain the Ea of System needed Activation Energy, Ea 1 4. (a) Identify each of the letters on the following generic energy diagram. Do not worry about letter “W”. Y = reactant(s) energy X = product(s) energy U = Ea T = ΔHRXN (b) Does this energy diagram represent an exothermic or an endothermic reaction? Explain. endothermic; the products are at a higher energy than the reactants 5. (a) Identify each of the letters on the following generic energy diagram. Do not worry about letter “C”. A = reactant(s) energy D = product(s) energy B = Ea E = ΔHRXN (b) Does this energy diagram represent an exothermic or an endothermic reaction? Explain based on the energy of the products. exothermic; the products are at a lower energy than the reactants 6. For each of the following energy diagrams, state whether the reaction is endothermic (EN) or exothermic (EX) A. B. A. and D. = Endothermic C. D. B. and C. = Exothermic 2 7. What is the rate of reaction? Rate of reaction = 𝒄𝒉𝒂𝒏𝒈𝒆 𝒊𝒏 𝒄𝒐𝒏𝒄𝒆𝒏𝒕𝒓𝒂𝒕𝒊𝒐𝒏 𝒄𝒉𝒂𝒏𝒈𝒆 𝒊𝒏 𝒕𝒊𝒎𝒆 = 𝜟 𝑴𝒐𝒍𝒂𝒓𝒊𝒕𝒚 𝜟𝒕 8. (a) What 3 things influence rate of reaction? I. II. III. Temperature Concentrations of reactants Catalysts (b) Explain how and why these three things influence rate of reaction. I. Temperature i. high T = reaction rate increases a. more average EK = more collisions b. more collisions with sufficient Ea ii. low T = reaction rate decreases a. less average EK = less collisions b. less collisions with sufficient Ea II. Concentration i. greater concentration = reaction rate increases a. more collisions ii. less concentration = reaction rate decreases a. less collisions III. Catalysts i. present = rate of reaction increases a. lowers Ea b. more collisions with sufficient Ea ii. absent = reate of reaction decreases a. less collisions with sufficient Ea 3 9. Indicate whether the following changes will increase, decrease, of have no effect upon the rate of reaction. Decrease (a) Decrease in concentration of reactants Decrease (b) Decrease in temperature Increase (c) Adding a catalyst 10. In the following reaction, what happens to the number of collisions when more Br2(g) molecules are added? H2(g) + Br2(g) → 2 HBr(g) WHAT: the number of collisions increases WHY: greater concentration of Br2(g) increases the chances that it will collide with H2(g) and cause a reaction 11. In the following reaction, what happens to the number of collisions when the temperature of the reaction is decreased? 2 H2(g) + CO(g) → CH3OH(g) WHAT: the number of collisions decreases WHY: lower temperature means lower EK = less collisions, and fewer of the collisions have sufficient Ea for a reaction 4 12. What is a catalyst? a substance that speeds up a reaction by offering an alternative pathway that has a lower Ea C. B. A. D. E. 13. (a) Match each of the following to the corresponding letter on the energy diagram shown above. C. i. Ea for the uncatalyzed reaction D. ii. ΔHRXN E. iii. energy of the products B. iv. Ea for the catalyzed reaction A. v. energy of the reactants (b) Is this reaction exothermic or endothermic? Explain based on the energy of the products. Exothermic, the products are at a lower energy than the reactants 5 14. How would each of the following change the rate of reaction shown here? Explain each. 2 SO2(g) + O2 (g) → 2 SO2(g) (a) Adding SO2(g) Rate of reaction increases b/c more collisions (b) Raising the temperature Rate of reaction increases b/c more EK per particle = more collisions, and more collisions have sufficient Ea (c) Adding a catalyst Rate of reaction increases b/c lower Ea means more collisions with sufficient Ea (d) Removing some O2(g) Rate of reaction decreases b/c less collisions 6 15. How would each of the following change the rate of reaction shown here? Explain each. 2 NO(g) + 2 H2 (g) → N2(g) + 2 H2O(g) (e) Adding NO(g) Rate of reaction increases b/c more collisions (f) Lowering the temperature Rate of reaction decreases b/c less EK per particle = less collisions, and less collisions have sufficient Ea (g) Removing some H2(g) Rate of reaction decreases b/c less collisions (h) Adding a catalyst Rate of reaction increases b/c lower Ea means more collisions with sufficient Ea 7