Practical project Group 3
advertisement

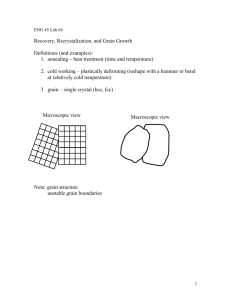
SYS862-1/Autumn 2014 Professor: Mohammad Jahazi Practical project Group 3 (Mahdi Masoumi, Keivan Heydari, Amir Keyvan Edalat Nobarzad,) Page 1 of 9 Contents 1 Introduction .................................................................................................................. 3 2 Situation of part as received ......................................................................................... 3 3 Experimental details ..................................................................................................... 4 3.1 Physical properties ................................................................................................ 4 3.2 Hardness ................................................................................................................ 4 3.3 Chemical composition ........................................................................................... 4 3.4 Metallography ....................................................................................................... 5 3.4.1 Analysis by optical microscope....................................................................... 5 3.4.2 SEM analysis ................................................................................................... 7 4 Discussion and conclusion............................................................................................ 8 5 Properties and application ............................................................................................ 9 6 References .................................................................................................................... 9 Page 2 of 9 1 Introduction The main goal of current project is to recognize the nature of an unknown part received related to the course of high strength materials. The method used in this project to obtain this recognition is reverse engineering. Following are some description to get more familiar with this method. Several professional organizations have provided the definitions of reverse engineering from their perspectives. The Society of Manufacturing Engineers (SME) states that the practice of reverse engineering “starting with a finished product or process and working backward in logocal fashion to discover the underlying new technology”. This statement highlights that reverse engineering focuses on process and analysis of reinvention in contrast to creation and innovation, which play more prominent roles in invention. Reverse engineering is a process to figure out how a part is produced, not to explain why this part is so designed. The functionality of the original part has already been demonstrated in most cases. The Military Handbook MILHDBK- 115A defines reverse engineering in a broader perspective to include the product’s economic value as “the process of duplicating an item functionally and dimensionally by physically examining and measuring existing parts to develop the technical data (physical and material characteristics) required for competitive procurement”. This definition casts light on the primary driving force of reverse engineering: competitiveness. The requested items in this project are listed below: • • • • • The materials The microstructure The properties The method of manufacturing Applications The selected methodology is to start from simplest and less expensive experiments to narrow down the hypothesis and then in case of necessity complementary tests where stablished to prove the hypothesis. 2 Situation of part as received The received part was a rectangular sample (75.7*43.7*19.1 mm) with code b4a2 in scripted on one side and the sides of parts have a surface as shown in Figure 2-1-a. Three circular traces were found on the top of part which same to be effect of spark, which is normally related to Mass Spectrometry test (Figure 2-1-b). Page 3 of 9 (a) (b) Figure 2-1. The situation of part as received a) in scripted code and cutting surface b) circular effect of sparks. 3 Experimental details 3.1 Physical properties The color of part was light gray, Density was determined as 7.75 gr/cm3; the part was submitted to a none destructive damping noise verification and no damping of noise and vibration was recognized. Magnetic property was checked by a magnet and it was proved that the part is attracted by magnet. 3.2 Hardness Rockwell-C hardness test was performed on different sides and in each side on different zones (totally 9 zones), the results were approximately same and the average was 33 HRc , this value is equal to 311Brinel. Top 33,6 33,5 32,9 33,3 0,309121 Hardness(HRC) Average Standard deviation Side 33,4 33,5 32,8 33,2 0,309121 Height 32,6 34,9 33,2 33,5 0,974109 3.3 Chemical composition Chemical composition of the part was detected by XRF method. The device was Olympus model Delta Premium and tube anode was Rh. the result are shown in Table 3-1 Table 3-1. The XRD result. 1 2 3 Average Standard Deviation Si 0.1531 0.1864 0.1616 0.167033 0.014127 Ni 0.4316 0.4409 0.4182 0.430233 0.009317 Cu 0.1464 0.1175 0.1532 0.139033 0.015477 Mo 0.5077 0.5122 0.5025 0.507467 0.003963 Page 4 of 9 V 0.1981 0.1708 0.1459 0.1716 0.021318 Mn 0.99 0.8857 1.0055 0.9604 0.053199 Cr 1.7965 1.8297 1.8177 1.814633 0.013726 3.4 Metallography 3.4.1 Analysis by optical microscope An investigation was performed using optical microscope to observe both microstructure and grain boundaries, two samples were cut from different sides of part as indicated in and were marked and samples A and B, both samples were etched with Nital %3 and microstructures were observed. The microstructure both samples (A and B) were the same, Figure 3-2 indicates the microstructure of sample A. (a) (b) Figure 3-1. Samples cut from different sides.(a) top surface,(b) side surface (a) (b) Figure 3-2. Microstructure of sample A etched by Nital 3% 10 Secconeds. a) 500X b) 1000X. As shown in Figure 3-2 the grain boundaries were not reveadled by Nital, therefore some others echants were test to reveal them, following are the list of tested etchants: • • • Nital+Picral Valella Kalling Page 5 of 9 None of above mentioned etchant revealed the grain boundaries; therefore a special etching procedure was applied: • • • 90 ml boiling distilled water 10 g picric acid Two drops of Zephiran chloride. The solution was kept in a temperature upper than 60 °C to avoid crystallization of picric. The samples were etched by above mentioned etchant (60s) followed by a polishing, this method is proposed by Mokhtar Ben Salah, Master student of ETS as shown in Figure 3-3. (a) (b) Figure 3-3 Preparation and etching of sample to reveal grain boundaries; (a) etching in solution (60S), (b) washing by Ethanol. Thereafter the grain boundaries in both samples (A and B) were observed, there was no difference between size and form of grains, Figure 3-4 indicates the grains of sample A. (a) (b) Figure 3-4. Grain boundaries revealed(a)specimen A,(b) sample B Page 6 of 9 3.4.2 SEM analysis To get more details about microstructure and also to reconfirm the amount of alloying elements an analysis was fulfilled by Scanning Electronic Microscope (SEM), the image shown in Figure 3-5 is the microstructure taken by SEM. Figure 3-6 indicates the chemical analysis obtained from a specific area by EDX. Figure 3-5. Microstructure of alloy using SEM etched by Nital 3% 10 Seconds. Figure 3-6. EDX analysis Page 7 of 9 4 Discussion and conclusion It is clear that the part is cut from a bigger plate, the marking on the part does not have any special meaning, and it seems that it is an internal coding of manufacturer. Verification of physical properties leads to get a general idea about the part, as mentioned some cut effects were detected on the parts, the color and density refers to a Ferro-alloy, however the noise damping verification proves that the part is likely to be steel rather than cast iron. As the part has magnetic property, the probability of being Austenitic steel is rejected. XRF analysis proposes ASTM A681, AISI P20 Steel, in spite of the fact that we don’t have %C but the chemical composition of this grade (proposed by XRF software) corresponds to chemical composition of sample part. The only difference is additional Vanadium which exists. It is assumed that the manufacturer added Vanadium to offer a sub-class to some customers with improved mechanical properties. The effect of Vanadium is to precipitate the vanadium-rich carbides during reheating, Vanadium carbide is categorized as a group 2 carbide in steels, group 2 carbides are not easily soluble in austenite event at high temperature, therefore the effect is pinning of grain growth of austenite and as a result the martensite derived from fine austenite will be also fine which leads to an increase in yield strength and toughness.[1] Because of specific usage of AISI P20 which will be explained in paragraph 5 the manufacturers always apply quench-temper heat treatment, the microstructure shown in Figure 3-2 and Figure 3-5 with a fine distribution of carbides confirms that the current sample also is tempered martensite. Figure 4-1 Shows the different grain orientations resulted by manufacturing processes, as there is no difference in grain orientations in top and side of the sample part Figure 3-4 it is obvious that the part is not cold rolled, as the grains are not columnal it can be concluded that the part is not casted. It is assumed that the part must be hot rolled or forged. (a) (b) (C) (D) Figure 4-1 Effect of manufacturing processes on grains;(a) Cold roll (b) casting (C)hot roll (D) Open die forge To conclude, the alloy is AISI P20 Steel plus an added amount of Vanadium which is produced by hot rolling or forge. Page 8 of 9 5 Properties and application Acceptable level of hardenability and also easy machining of AISI P20 Steel makes it suitable to be used for tools like injection molds, extrusion dies and compression moulds. Furthermore excellent weldability of this steel gives it the repairable by welding which is crucial for injection molds. In case of forging, the proposed starting temperature is 1010 to 1120̊C, it shouldn’t be forged below 870̊C, Normalizing is possible at 900̊C, to be used as a tool steel it is usually quenched-tempered. [2] Neglecting of effect of Vanadium existed in concerned alloys Figure 5-1 can be considered to estimate the temperature which the sample part is tempered, considering the hardness mentioned in paragraph 3.2 (33HRC) the tempering maybe be done in a high temperature(>600̊C), while the proposed range for AISI P20 is 480̊C to 595̊[2] another hypothesis which is more probable(because the manufacturers knows well about the tempering range) is that the part maybe tempered for a longer time. It can be assumed that the sample part is cut from the core of a big slab which leads the core to be tempered in a long time compared to surface and obtaining a hardness lower than required for quenched-tempered AISI P20. Figure 5-1 Hardness vs Austeniting and tempering temperature, P20[2] 6 References 1. 2. Yang, G., et al., Effects of vanadium on the microstructure and mechanical properties of a high strength low alloy martensite steel. Materials & Design, 2013. 50(0): p. 102-107. Chandler, H., Heat Treater's Guide: Practices and Procedures for Irons and Steels. 1994: ASM International. Page 9 of 9