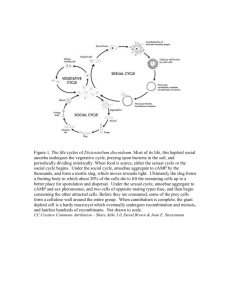
Tetraspanin CD151 is involved in control of stem cell
advertisement

Analysis of morphological and molecular signature of invasiveness in breast ductal carcinoma in situ (DCIS) Magdalena Mieszkowska Laboratory of Molecular Enzymology Support thesis supervisor: dr Rafał Sądej Thesis supervisor: prof. dr hab. Andrzej C. Składanowski Breast cancer (BCa) is the leading cause of women death worldwide. Human mammary ductal carcinoma originates from epithelial hyperproliferation, evolves into in situ (DCIS) and invasive carcinomas (IDC), to finally become a metastatic disease. DCIS, non-invasive breast cancer, is a highly heterogeneous precursor of IDC. Recent studies have implicated a number of genes in development of DCIS but so far, there are no markers which would reliably identify the initial steps of its progression into invasive disease. Increasing evidence suggests that SLUG, a transcription factor also known as SNAIL2, is involved in invasiveness of human cancers. Overexpression of SLUG in basal and mesenchymal subtypes correlates with poor prognosis and shorter overall survival of the patients with aggressive BCa. Moreover, increased SLUG expression resulted in downregulation of HER2 in the cells. Importantly, HER2 is found in more cases of DCIS (50-60%) as compared to HER2-positive invasive tumours (2025%). Therefore in this project we investigate interdependence between HER2 and SLUG in DCIS progression into invasive breast cancer. We hypothesize that two mechanism might be responsible for this progression: - in HER2-negative cells - proliferative dominance of a subpopulation of basal-like cells existing in a pre-invasive DCIS lesion - in HER2-postive cells - increased expression and activity of SLUG leading to downregulation of HER2 and a subsequent emergence of a basal-like subclone displaying stem cell-like traits Our in vitro model consists of two cell line: HB2 (luminal epithelial cells, DCIS-like lesions model in vivo) and MCF10A (basal epithelial cells). Their SLUG and HER2 – overexpressing or knock down variants were developed with retroviral or lentiviral systems. Role of SLUG in HER2 expression was assessed by western blotting. Cell growth and induction of an invasive phenotype of generated mutant cell lines vs wild type were assessed in 3D cultures. The preliminary results demonstrate that overexpression of ErbB2 and SLUG induces morfological alteration in MCF10A in comparison to wild type. Moreover high level of SLUG modulates invasiveness in basal/stem cell-like breast cancer. We found that SLUG upregulated or downregulated ErbB2 and SOX9 expression depending on the cell line. These results suggest that not only one marker is sufficient to drive DCIS to IDC evolution. Identification of the key mechanism underlying of the DCIS progression towards IDC in vitro and in vivo will provide biological insight in the search for the new diagnostic markers to improve tumour prognostication.