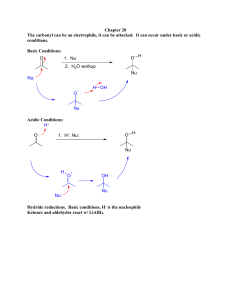
Addition Reactions
advertisement
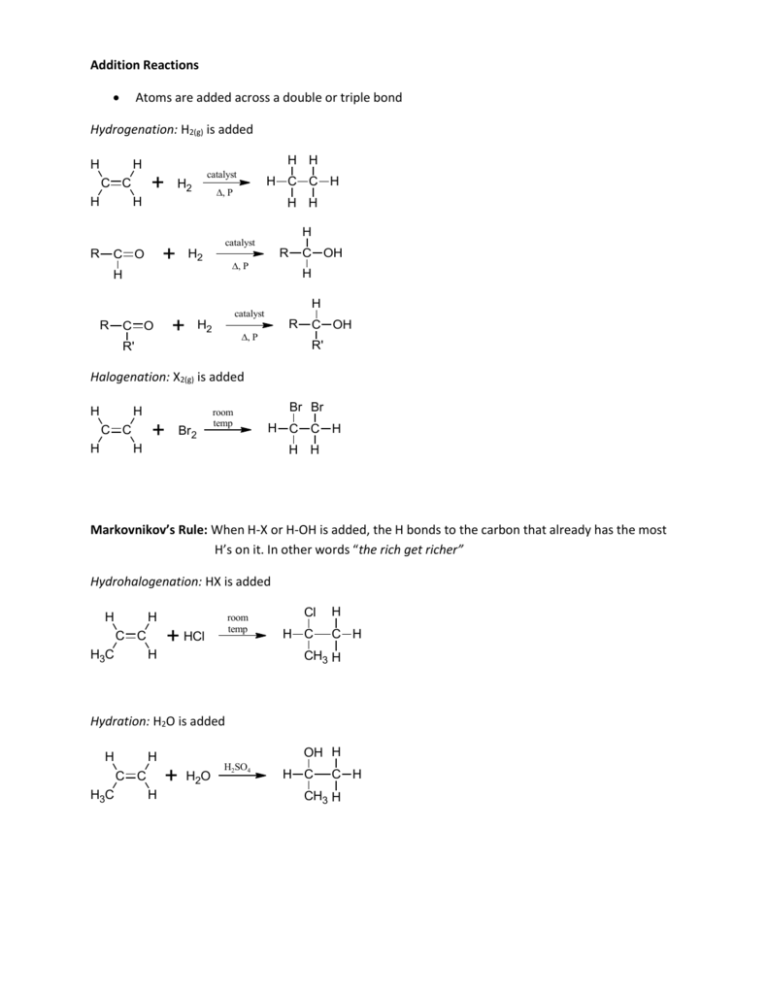
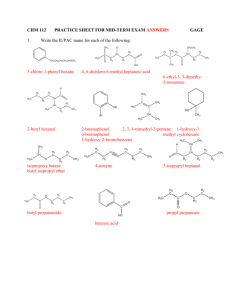
Addition Reactions Atoms are added across a double or triple bond Hydrogenation: H2(g) is added H H H H + C C H catalyst H2 H R H H H2 R , P H C OH H H catalyst + C O H catalyst + C O R H C C H , P H2 R , P R' C OH R' Halogenation: X2(g) is added H H + C C H Br2 room temp Br Br H C C H H H H Markovnikov’s Rule: When H-X or H-OH is added, the H bonds to the carbon that already has the most H’s on it. In other words “the rich get richer” Hydrohalogenation: HX is added H H + HCl C C H3C room temp H Cl H C H C H CH3 H Hydration: H2O is added H + C C H3C OH H H H H2O H2SO4 H C C H CH3 H Substitution Reactions Hydrogen atom is replaced by another atom or group of atoms Halogenation: Halogen replaces a hydrogen when X2(g) or HX is added H H H H Br + C C H HBr or h H H H + C C H H2 H H Cl + Cl Cl FeBr3 + H Cl Nitration: NO2 replaces a hydrogen when HNO3 is added + HNO 3 NO 2 H2SO4 + H2O Alkylation: An alkyl group replaces a hydrogen when RX is added + H3C Br H N H + CH3 AlCl3 + HBr H H3C Cl H N CH3 H + HCl CH3 H N CH3 + H3C H2C CH2 Cl H N CH3 + HCl H CH3 H2C N CH3 H CH3 + H2C H3C Cl N CH3 H3C + HCl Elimination Reactions Results in loss of small molecule from larger molecule which causes a single bond to become a double bond, and double to become a triple When molecule is not symmetric, isomers occur. The major product is the one where the hydrogen is removed from the carbon with the most carbon-carbon bonds Dehydration: Water is formed as a product H H H H R C C R' + OH - R C C R' + H2O + Cl - Cl H H H H H R C C R' acid R C C R' + H2O H OH Condensation Reactions Two molecules combine for form a larger product, eliminating a small molecule such as water or alcohol Dehydration: Water is formed as a product, removing an –OH group from one molecule and an H from the other molecule where the 2 molecules are joined R OH + R' H2SO4 OH R O R' O O H3C CH2 C OH + H2N CH3 H3C CH2 C NH CH3 + H2O Esterification: Reaction of a carboxylic acid with an alcohol to form an ester and water O O H3C CH2 C OH + H3C OH H2SO4 H3C CH2 C O CH3 + H2O Controlled Oxidation Reactions Gain of oxygen or loss of hydrogen (O) tells us that the oxygen is supplied by an oxidizing agent K2Cr2O7 or KMnO4 in the presence of H2SO4 H OH H H O C C H + (O) H H H C C + H2O H H Note that the aldehyde can also be oxidized further into a carboxylic acid OH H3C C O CH3 + (O) H3C C CH3 + H2O H Note that the ketone can not be oxidized since there is no H on the C to make room for a double bond. OH H3C C CH3 + (O) NO REACTION CH3 Hydrolysis Water molecules are split into H+ and OH- and are used to break down a molecule into smaller molecules Sometimes an acid or base can be used instead of water (in cation and anion form) o When this is done with esters, the sodium salt of the acid that results is soap o This process is called saponification O O H3C CH2 C O CH3 + + Na O H3C CH2 C + OH - H3C CH2 C - + O Na + H3C O O NH2 + H2O H3C CH2 C OH + H2N OH OH Reduction R Reaction in which carbon atom forms fewer bonds to oxygen atoms or more [H] indicates a reducing agent, usually LiAlH4 or H2 with a Pt catalyst + [H] C O R' H H + HCl C C H3C room temp H Combustion Among most common oxidation-reduction reactions Complete Combustion: Excess of oxygen allows reactants to completely react Hydrocarbon + O2(g) CO2(g) + H2O(g) + energy Incomplete Combustion: Occurs when insufficient oxygen is present Hydrocarbon + O2(g) C(s) + CO(g) + CO2(g) + H2O(g) + energy