Formal Lab 2 H2O2 Redox Titration
advertisement

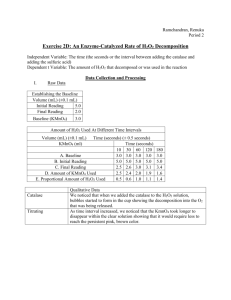
Formal Lab #2: H2O2 Redox Titration Many times we pick up a chemical solution at the local pharmacy or grocery store, and do not think twice about the concentration of the substance inside. Over time the concentration of solutions can change. For example, if we have a solution that was prepared at 5% that has sat on a shelf or in storage for a very long time, that concentration may not be only 3.9%. In this activity, you will determine the concentration of commercially-available hydrogen peroxide. The label states that the solution inside the bottle is 3% hydrogen peroxide. You will determine if this is truly the case. In order to determine the actual working concentration of the hydrogen peroxide, you will first have to standardize a solution. You will perform a redox titration. In an oxidation-reduction reaction the equivalence point occurs when the quantity of titrant added is the exact amount necessary for stoichiometric reaction with the analyte. In the equation shown below, this occurs when 5 moles of oxalic acid react with exactly 2 moles of permanganate in hot acidic solution. 5 H2C2O4 (aq) + 2 MnO4-(aq) + 6 H+(aq) → 10 CO2(aq) + 8 H2O(l) + 2 Mn2+(aq) Although the equivalence point is the ideal stopping point of a reaction, it is not often what is measured. The end point is often observed in a titration reaction because it marks a sudden change in a physical property of the solution. In the equation above, this end point is signified by a change in color of the solution from colorless to purple. This indicates when all the permanganate is consumed by the oxalic acid, and excess permanganate ions remain. Your formal lab report will include each of the following: ● Cover page (see last page of this packet) ● Prelab Questions—answers must be written in full sentences, all work must be shown ● Data tables and calculations ● Postlab Questions—answers must be written in full sentences ● Conclusion (typed) Prelab Questions 1) The skeleton unbalanced equation for the reaction of permanganate with hydrogen peroxide is: MnO4- + H2O2 Mn2+ + O2 + H2O a) Write the balanced half reaction for the reduction of permanganate ions in solution. b) Write the balanced half reaction for the oxidation of hydrogen peroxide. c) Write the balanced equation for the reduction of permanganate by hydrogen peroxide. 2) You are going to standardize your permanganate solution using iron (II). The skeleton unbalanced equation for this reaction is MnO4- + Fe2+ → Fe3+ + Mn2+ a) Write the balanced half reaction for the reduction of permanganate ions in solution. b) Write the balanced half reaction for the oxidation of iron(II) to iron(III). c) Write the balanced equation for the reduction of permanganate by iron(II). 3) Using a standardized solution of KMnO4, 30.0 mL of oxalic acid, H2C2O4, was titrated until a faint pink color was observed. This pink color was observed after approximately 15.59 mL of 0.0300 M KMnO4 was added. The balanced equation for this reaction is 5 H2C2O4 (aq) + 2 MnO4-(aq) + 6 H+(aq) → 10 CO2(aq) + 8 H2O(l) + 2 Mn2+(aq) a) How many moles of MnO4- ions reacted during this reaction? b) How many moles of H2C2O4 are present in this reaction? c) What is the molarity of the oxalic acid solution? Procedure: fill out this section and attach it, along with any work required, to the back of your lab report 1) Obtain a clean flask and a clean buret. Rinse out your buret with distilled water, then flush it with a few mL of KMnO4 by adding a few mL to it and draining it out through the stopcock. 2) Weigh out 1-2 g of iron (II) nitrate. Record this mass accurately here: __________________g 3) Put the iron (II) nitrate in a flask and add enough distilled water to dissolve it. Set up the Fe(NO3)2 for titration with your KMnO4 solution. Record the original KMnO4 volume here: _______________ mL. Add about 3 mL of sulfuric acid to the iron (II) nitrate to acidify it. The end point for this titration is a faint pink color. Record the final KMnO4 volume here: _______________ mL 4) Record the volume of KMnO4 required to titrate the iron (II) nitrate here: _____________ mL. Use this information to determine the concentration of your KMnO4 solution. Show all work on a separate piece of paper. 5) Add more KMnO4 to your buret if you think it is necessary. Record the original KMnO4 volume here: _______________ mL 6) Obtain a clean flask or beaker. Rinse with distilled water and wipe dry with a paper towel. Add 1.00 mL H2O2 and 3.00 mL H2SO4 to this flask/beaker. 7) Titrate your acidified H2O2 to the end point with KMnO4. The end point for this titration is a faint pink color. Record the final KMnO4 volume here: _______________ mL. Record the volume of KMnO4 required to titrate your hydrogen peroxide here: _________________ mL 8) Determine the molarity of your hydrogen peroxide solution. Show all work on a separate piece of paper. 9) Calculate the mass percent of your hydrogen peroxide solution. Show all work on a separate piece of paper. a. Mass of hydrogen peroxide = molarity of H2O2 x .00100 L x molar mass of H2O2 b. Mass of water = 1.00 mL x 1.00 g/mL = 1.00 g water c. Mass percent = (mass of hydrogen peroxide/mass of water) x 100% Data Analysis Perform all calculations indicated on your cover page and listed above. Show all work. Make sure to record each final value on your cover page (this should be the absolute last thing you do on this lab). Postlab Questions--don’t write the question, but fully explain yourself 1) Compare the mass % of hydrogen peroxide that you calculated in this lab to the percent on the bottle. 2) What is the difference between a titrant and an analyte? 3) Why was it necessary to standardize the solution before performing this titration to determine the concentration of hydrogen peroxide? Conclusion Comment on the discrepancy between the mass percent of hydrogen peroxide that you calculated in this lab with the percent labeled on the bottle. Explain why your number is higher or lower than the bottle states. This formal lab report is a major grade. Each student needs to submit his or her own report. Plagiarism and copying will not be tolerated. Formal Lab #2: H2O2 Redox Titration Due M 12/1/2014, not accepted late, +5 points if turned in by F 11/21/2013 Submitted by ___________________________ Group Members: _______________________________________________________________________ Purpose of Lab ________________________________________________________________________ _____________________________________________________________________________________ Standardization of KMnO4 Balanced equation for the reduction of permanganate by iron(II): Mass of FeSO4 Moles of FeSO4 Volume of KMnO4 Moles of KMnO4 Molarity of KMnO4 Concentration of H2O2 Balanced equation for the reduction of permanganate by hydrogen peroxide: Volume of H2O2 Solution 1.00 mL Volume of 3 M H2SO4 3.00 mL Volume of KMnO4 Moles of KMnO4 Moles of H2O2 Molarity of H2O2 Mass of H2O2 (calculated from above mass) Mass of H2O in H2O2 solution Percent mass of H2O2 in solution